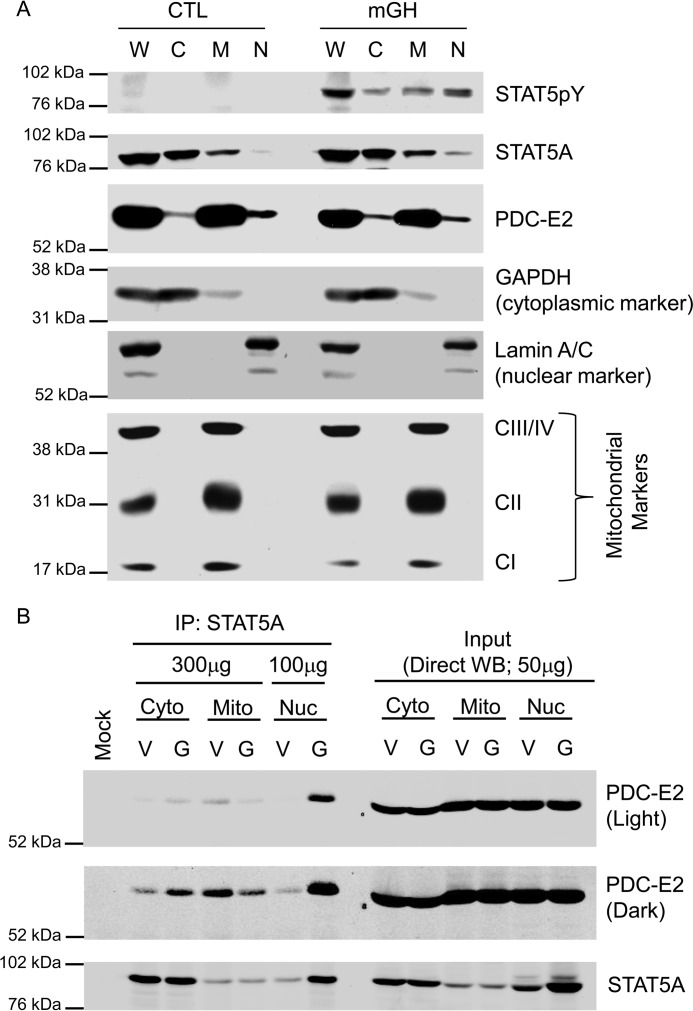
Figure 7.
PDC-E2 is present in adipocyte nuclei where it associates with activated STAT5A. Mature 3T3-L1 adipocytes were serum-deprived overnight and then treated with 5 nm mGH (G) or vehicle (V) for 20 min. Control (CTL) plates were untreated. A, monolayers were detached using trypsin and then subjected to subcellular fractionation using a cytoplasmic, nuclear, and mitochondrial fractionation kit. Cytoplasmic (C), mitochondrial (M), and nuclear (N) fractions from each treatment group were analyzed by immunoblotting. A whole-cell lysate (W) of each treatment group was also prepared separately and analyzed alongside the fractions. CI (20 kDa), CII (30 kDa), and CIII/IV (40/48 kDa) indicate subunits of the electron transport chain complexes that were immunoblotted as mitochondrial marker proteins. GAPDH (37 kDa) and Lamin A/C (62/69 kDa) were used as cytoplasmic and nuclear markers, respectively. B, monolayers were scraped into nuclear homogenization buffer and subjected to subcellular fractionation using differential centrifugation. The cytoplasmic (Cyto), mitochondrial (Mito), and nuclear (Nuc) extracts from each treatment group were subjected to immunoprecipitation using an anti-STAT5A antibody. The mock experiment contained IP antibody but no extract. The amount of total protein used in each IP is indicated in the figure. Western blotting was used to examine the protein content of the immunoprecipitates (IP: STAT5A; left) and subcellular fraction inputs (Direct WB; 50 μg of protein/lane; right). Fractionation results were replicated at least three times on independent batches of adipocytes.