Figure 2.
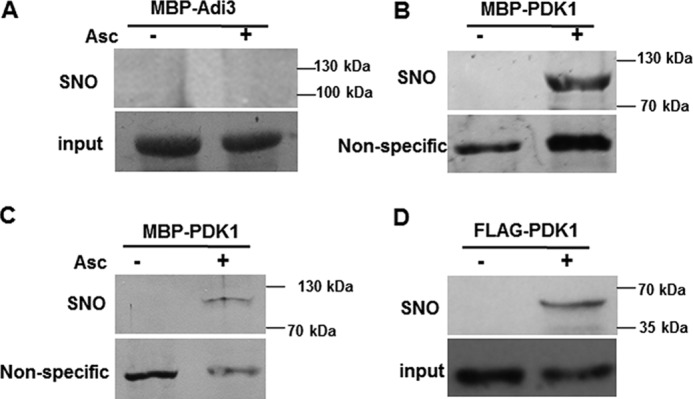
SlPDK1 is a target of S-nitrosylation. A and B, MBP–Adi3 (A) and MBP-SlPDK1 (B) were purified from bacterial extracts and treated with 1 mm GSNO. S-nitrosylation was detected by biotin-switch assay using a biotin antibody. Asc, ascorbate. C, biotinylated MBP–SlPDK1 protein was purified with neutravidin–agarose, separated by SDS-PAGE, and detected by immunoblot assay with an anti-MBP antibody. Coomassie Blue was used to stain MBP–Adi3 protein for loading control in A (input), and the nonspecific bands detected in immunoblots were used as controls in B and C. D, the FLAG–SlPDK1 fusion protein transiently expressed in the leaves of NbGSNOR1-silenced N. benthamiana plant is S-nitrosylated in vivo. Equal loading was verified with FLAG antibody against the input protein extracts. These experiments were repeated three times with similar results.
