Figure 4.
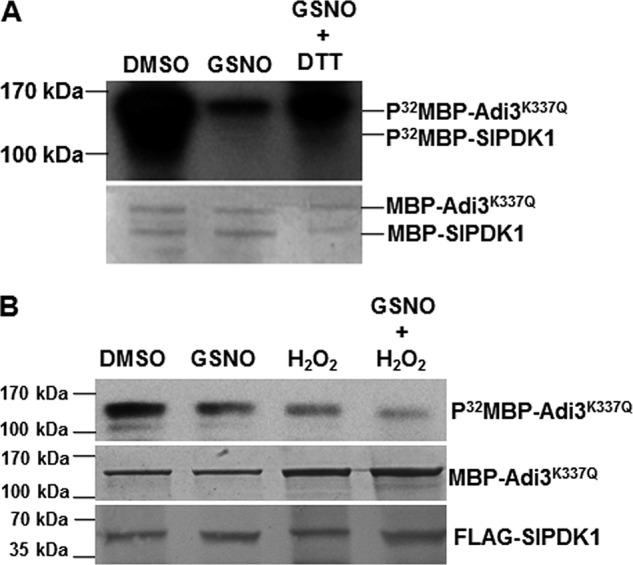
Inhibition of SlPDK1 kinase activity is reversible by reducing agent in vitro but enhanced additively by H2O2 when transiently expressed in N. benthamiana leaves. A, DTT partially reversed the inhibitory effect of GSNO on trans-phosphorylation of MBP–Adi3 K337Q by MBP–SlPDK1 in vitro. The Coomassie Blue–stained gel is shown for loading control. B, the kinase activity of SlPDK1 transiently expressed in the leaves of N. benthamiana plants was additively inhibited by 2 mm GSNO and 1 mm H2O2. 35S::FLAG-SlPDK1 was transiently expressed in N. benthamiana via agroinfiltration. The kinase activity of the cell extracts from the infiltrated area was determined using purified kinase-dead MBP-Adi3K337Q as a substrate. Equal loading for MBP–Adi3 K337Q was shown by Coomassie Blue staining, and equal input for FLAG–SlPDK1 was shown by Western blot analysis using FLAG antibody. The experiments were repeated three with similar results.
