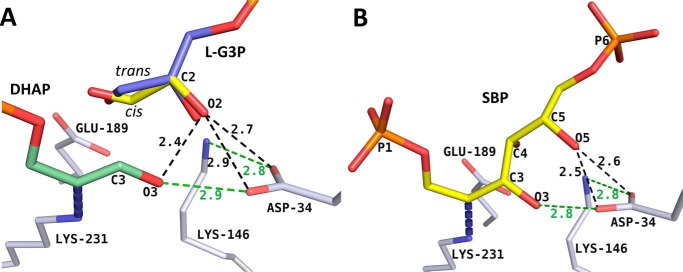
Figure 4.
l-G3P model reveals basis for enantiomeric discrimination in class I aldolase. A, the l-enantiomer of the bound d-G3P (yellow) was superimposed on the d-G3P geometry from the 1-min structure. Favorable linear syn hydrogen bonds are depicted as green dashes. The steric repulsion resulting from proximity of l-G3P O2 to surrounding oxygens (DHAP O3 oxygen, Asp-34 carboxylate oxygens) is depicted as dark dashes. The trans-configuration (blue) for l-enantiomer modeled using the trans-d-G3P model yields repulsions comparable with those observed for the cis-configuration. B, SBP was modeled into the active site using the FBP Schiff base intermediate as a template. Clashes (dark dashes) and favorable hydrogen bonds (green dashes) are illustrated.