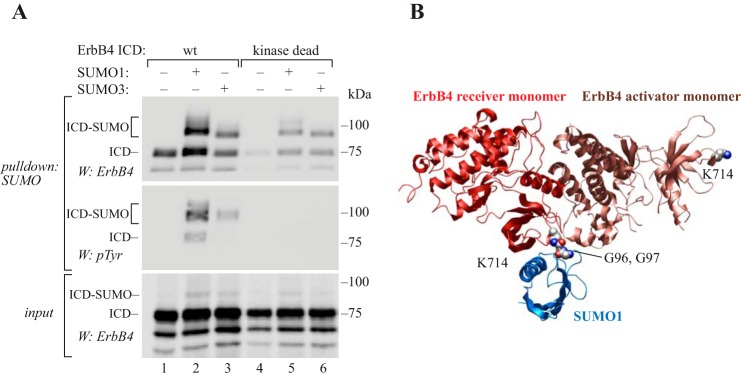
Figure 6.
SUMOylation increases autophosphorylation of ErbB4 ICD. A, COS-7 cells were transfected with wild-type or kinase dead K751R ErbB4 ICD2 with or without His6-tagged SUMO constructs as indicated. Cells were lysed in denaturing buffer, and lysates were incubated with Ni2+-NTA agarose to pull down His6-SUMO conjugates. Whole cell extracts were analyzed by Western blotting with anti-ErbB4, and pulldown samples with anti-ErbB4 or anti-phosphotyrosine (4G10, Upstate). Representative data of two independent experiments are shown. B, structural model (displayed as secondary structures) of the covalent complex between the ErbB4 kinase asymmetric dimer X-ray structure (RCSB PDB ID: 3BCE) and an NMR structure for SUMO1 (in blue) (RCSB PDB ID: 2ASQ). Lys-714 (CPK representations) in both the activator and receiver kinase domains are indicated but SUMO1 is shown ligated only to Lys-714 of the receiver kinase via an isopeptide bond represented between the lysine side chain and the C-terminal Gly-Gly sequence of SUMO (CPK representations).