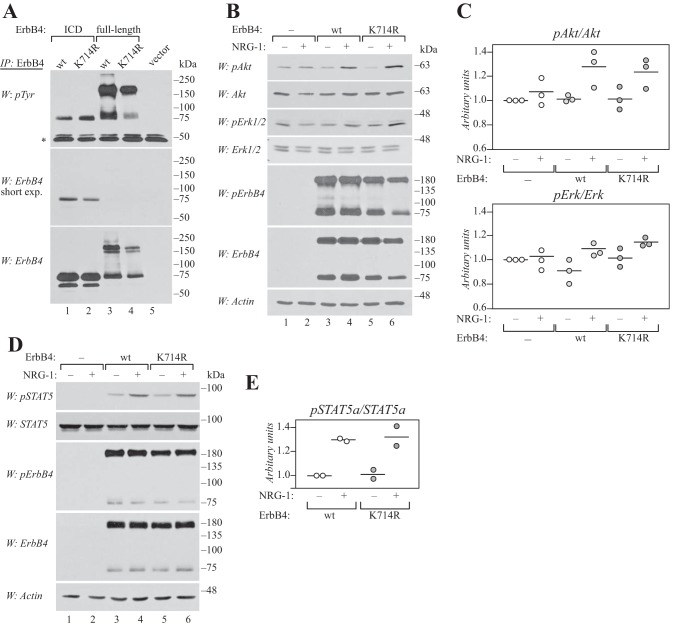
Figure 7.
Lys-714 is not required for signaling of full-length ErbB4 at the cell surface. A, COS-7 cells expressing wild-type or K714R ErbB4 ICD2 or JM-a CYT-2 were starved overnight without serum, lysed, and immunoprecipitated (IP) with anti-ErbB4. Phosphorylation was analyzed by Western blotting with anti-phosphotyrosine (4G10, Upstate), and the membrane was reprobed with anti-ErbB4. Representative data of five independent experiments are shown. *, IgG heavy chain. B, COS-7 cells expressing wild-type or K714R ErbB4 JM-a CYT-2 were starved without serum overnight, stimulated for 10 min with 50 ng/ml NRG-1, and lysed. Phosphorylation of Akt, Erk1/2, and ErbB4 was analyzed by Western blotting with phospho-specific antibodies. Loading was controlled using antibodies recognizing total Akt, Erk1/2, ErbB4, or actin. Representative data of three independent experiments are shown. C, quantification of pErk1/2 and pAkt signal intensities normalized to total Akt and Erk. Data are presented as scatter plots, with circles indicating data points from three independent experiments and horizontal lines indicating the mean. D, COS-7 cells expressing wild-type or K714R ErbB4 JM-a CYT-2 together with STAT5a were treated as in B. Phosphorylation of STAT5 and ErbB4 was analyzed by Western blotting with phospho-specific antibodies. Loading was controlled using antibodies recognizing total STAT5, ErbB4, or actin. Representative data of two independent experiments are shown. E, quantification of pSTAT5a signal intensity normalized to total STAT5a. Data are presented as a scatter plot, with circles indicating data points from two independent experiments and horizontal lines indicating the mean.