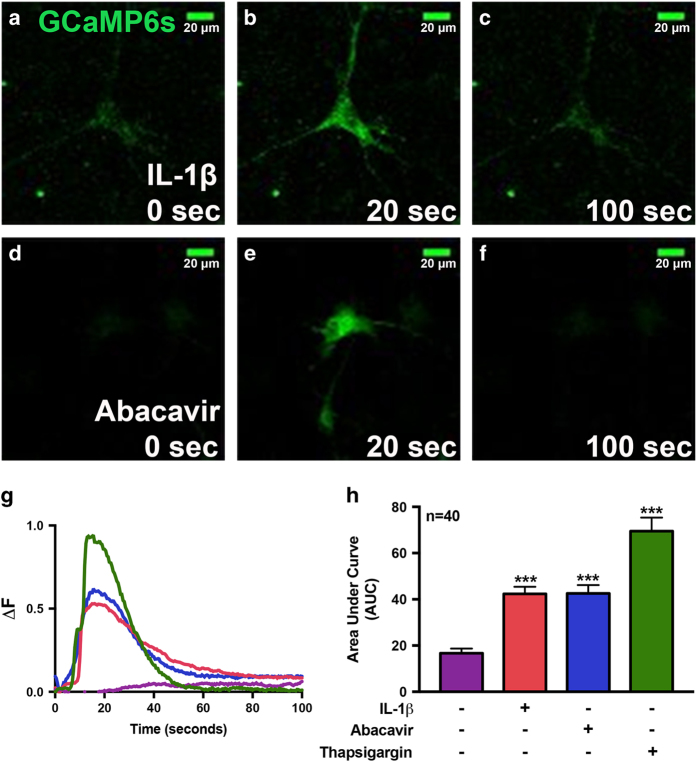
Figure 4.
IL-1β and abacavir induce intracellular calcium signaling in human astrocytes. For analysis of calcium signaling, primary human astrocytes were first transfected with GCaMP6s, a plasmid expressing an ultrasensitive protein calcium sensor, and allowed to recover for 48 h. Transfected cells were treated with IL-1β (20 ng/ml, a–c) and abacavir (4 μM, d–f). Fluorescence was visualized by confocal microscopy and images were captured every 500 ms. (a–f) Fluorescent images taken from a representative cell at time 0 (a and d, before treatment) and at 20 (b and e) and 100 s (c and f), post IL-1β and abacavir treatment, respectively. The histogram (g) shows fluorescence intensity ratio (ΔF) of the representative astrocyte captured over the entire imaging period before and after treatments with HBSS (control, violet), IL-1β (orange), abacavir (blue), and Th (200 nM, green). Bar graph (h) denotes intracellular Ca+2 increase, quantified as area under the curve (AUC) in IL-1β-, abacavir-, and Th-treated astrocytes as compared to control. Cumulative data from three individual donors are shown. Data represent mean±S.E.M., and statistical analyses were performed using one-way ANOVA with Tukey’s post-test for multiple comparisons (***P<0.001, n=40 individual cells/treatment).