Abstract
Background
Depression frequently co-occurs with cognitive decline, but the nature of this association is unclear. We examined relations of late-life depressive symptom patterns to subsequent domain-specific cognitive changes.
Methods
Depressive symptoms were measured at up to 3 timepoints among 11,675 Nurses’ Health Study participants prior to cognitive testing. Depressive symptom patterns were categorized as non-depressed, variable, or persistent, based on published severity cutpoints. Outcomes were global, verbal, and executive function-attention composite scores.
Results
Participants with persistent depressive symptoms had worse executive function-attention decline compared to non-depressed participants (multivariable-adjusted mean difference=-0.03 units/year, 95% CI: −0.05, −0.01; p=0.003); this difference was comparable to 8 years of aging. However, being in the persistent vs. non-depressed group was not significantly related to verbal (p=0.71) or global score (p=0.09) decline. By contrast, compared to the non-depressed group, those with variable depressive symptoms had worse verbal memory decline (multivariable-adjusted mean difference=−0.01 units/year, 95% CI: −0.02, −0.002; p=0.03); this group showed no differences for global or executive function-attention decline.
Conclusions
A variable pattern of depressive symptom severity related to subsequent decline in verbal memory, while a persistent pattern related to decline in executive function-attention. Findings could signal differences in underlying neuropathologic processes among persons with differing depression patterns and late-life cognitive decline.
Keywords: Cognitive decline, cognitive domains, depression, patterns of depression, aging, dementia
1. INTRODUCTION
Depression and cognitive impairment, including dementia, frequently co-occur in older adults.(Zubenko et al., 2003, Olin et al., 2002) There are several possible explanations for this high degree of comorbidity. Late-life depression may be an independent risk factor for cognitive decline or may represent a prodromal phase of dementing illness.(Amieva et al., 2008)
Conflicting evidence exists regarding the influence of depression on subsequent cognition and dementing illnesses. Some research indicates that depression may increase risk of cognitive decline. In one study, older adults with baseline depression showed greater hippocampal atrophy over follow-up and this reduction in volume was associated with worse cognitive-decline.(Steffens et al., 2011) Further, lifetime history of depression increases risk of dementia and recurrent depressive episodes monotonically increase this risk.(Van Duijn et al., 1994, Dotson et al., 2010)
Other studies provide evidence in favor of a prodromal contribution of depression to cognitive decline and dementia. A study of older twins (Steffens et al., 1997) found that history of a major depressive episode was associated with an increased risk of Alzheimer disease (AD). However, as the time between the onsets of the depressive episode and dementia increased, the risk of developing dementia decreased - indicating that these depressive symptoms may reflect prodromal AD. Additionally, in a study of hospitalized older adults with depression, those with co-morbid depression and dementia had a later age of first depression onset compared to those with depression alone.(Alexopoulos et al., 1993)
If late-life depression (LLD) is a prodromal indicator of underlying neuropathology, then differences in patterns of antecedent depressive symptoms may relate to different sub-types of cognitive decline (e.g., amnestic vs. dysexecutive). However, few studies(Paterniti et al., 2002, Singh-Manoux et al., 2010, Kohler et al., 2010, Graziane et al., 2016) have examined how LDD patterns relate to subsequent cognitive decline by domain. In one example (Paterniti et al., 2002) cognitively normal older adults with persistent, but not variable, depressive symptoms showed greater cognitive decline compared to those with no depression; however, domain-specific associations were not examined. Domain-specific cognitive-functioning was examined (Singh-Manoux et al., 2010) among 4271 participants aged 35-55 years followed for 18 years: persistent depressive symptoms in late-midlife, compared to no symptoms, increased odds of worse cognitive function for all domains. However, cognition was assessed only at the end of follow-up, and information was unavailable regarding how depression patterns related prospectively to cognitive trajectories; furthermore, the young age of participants limited applicability to the question of how LLD patterns relate to subsequent cognition. In a study concurrently examining depression and cognition over 6 years (Kohler et al., 2010), older adults with persistently-high depressive symptoms showed worse decline in memory, processing-speed and global functioning, compared to those who never had high depressive symptoms. Another study (Graziane et al., 2016) examined dual trajectories of depression and cognitive function simultaneously over 5 years: worsening, low-grade depression was related to persistently low attention, while moderate depression was related to persistently low executive, language and memory. However, the simultaneous assessment of depression and cognition in these studies limited the ability to relate LLD patterns to subsequent cognitive outcomes.
The field can benefit from studies addressing how patterns of LLD symptoms relate to subsequent cognitive change – both globally and within domains. Thus, we related patterns of LLD symptoms, measured up to 3 times over 8-years among 11,675 older women in the Nurses’ Health Study (NHS), to subsequent change in cognitive domains, serially assessed up to 4 times over an average of 5.5 years.
2. METHODS
2.1 Study sample
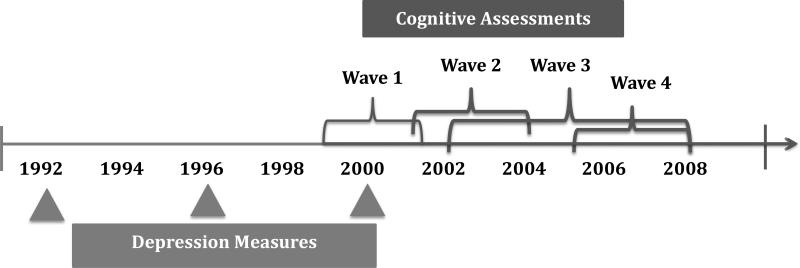
In 1976, 121,700 female nurses aged 30 to 55 years in 11 U.S. states were enrolled in the NHS. Participants have completed mailed questionnaires every two years since on lifestyle, behavioral and health-factors and medical-outcomes. Follow-up has remained at approximately 90%. In 1992, 1996 and 2000, participants completed the Mental Health Inventory–5 (MHI-5) sub-scale from the Medical Outcomes Study Short-form-36.(Ware and Sherbourne, 1992) Additionally, between 1995-2000 (Figure 1) those participants aged 70+ years without history of stroke were invited to enroll in a cognitive function sub-study. Of those invited to participate in this sub-study, 93% completed the initial interview. Three additional waves of cognitive follow-up have been completed.
Figure 1.
Timeline of Depressive Symptom Measures and Cognitive Assessments
The exposure is pattern of depressive symptoms, measured every 4 years by the MHI-5 between 1992-2000. To be included in the analysis, participants must have completed at least two MHI-5 questionnaires prior to initial cognitive-testing. Of the initial cognitive sub-study population of 19,415 women, 11,675 were included in our study due to exclusions for missing or insufficient depression questionnaire responses Cognitive sub-study participants excluded from the analysis had lower physical activity (15.0 vs 16.2 metabolic-equivalent task hours/week) and were more likely to be current smokers (10% vs 7%). They were comparable in age (74.3 vs 74.2 years) and proportion using antidepressants (5% vs 6%). Excluded participants also did not differ from those included in reported alcohol consumption, BMI or number of comorbidities. The Institutional Review Board of Brigham and Women's Hospital, Boston, MA, approved this study.
2.2 Ascertainment of depression
Depressive symptoms were measured using the MHI-5 contained within NHS questionnaire. The MHI-5 consists of five questions on frequency of symptoms (3 address depression, 2 address anxiety) over the past four-week period, with six response options of: all, most, a good bit, some, a little, or none of the time.(Cuijpers et al., 2009) It has been validated for detection of depression.(Berwick et al., 1991, Rumpf et al., 2001, Friedman et al., 2005) MHI-5 scores range from 0 to 100, where lower scores indicate more severe symptoms. As done elsewhere, we used the cut-point of ≤52 to denote presence of severe depressive symptoms.(Holmes, 1998, Bultmann et al., 2006)
Since the objective was to relate LLD symptoms to subsequent cognitive decline, we only included MHI-5 responses prior to the start of cognitive testing. In our sample, 86% of participants returned two questionnaires and 14% returned three questionnaires prior to initial cognitive interview. Depressive symptom patterns were categorized in three ways using the specified cut-point on the MHI-5: non-depressed, variable, and persistent.(Holmes, 1998, Bultmann et al., 2006) If a participant reported MHI-5 scores >52 at all timepoints, she was assigned to the “non-depressed” group; if she scored ≤52 at all timepoints, she was assigned to the “persistent” group; finally, if she scored ≤52 at one or more timepoints, but not all, she was assigned to the “variable” group. The number of timepoints used to characterize pattern was based on entry date into the cognitive sub-study. For example, if a participant's initial cognitive interview occurred prior to the return of her 2000 NHS questionnaire, then only her 1992 and 1996 MHI-5 scores were used to categorize depressive symptoms; whereas if a participant's 2000 questionnaire was returned prior to her initial cognitive interview, then her 1992, 1996 and 2000 MHI-5 responses were used. The number of depression assessments completed prior to the initial cognitive testing differed between groups; two depression assessments were completed prior to the first wave of cognitive testing among 86%, 79%, and 93%, respectively, of the non-depressed, variable and persistent depression groups. However, the number of assessments used to categorize depression is expected to be unrelated to outcome; therefore, any bias related to these differences would be non-differential. In order to verify this, we conducted a sensitivity analysis in which we categorized the entire sample based only on the 1992 and 1996 depression questionnaires and compared results to our main analyses.
2.3 Ascertainment of cognitive function
Cognitive function was assessed using a telephone-based method described elsewhere.(Okereke and Grodstein, 2013, Stampfer et al., 2005) Briefly, beginning in 1995 participants were administered the Telephone Interview for Cognitive Status (TICS), assessing general cognition and comparable to the Mini-Mental State Examination.(Brandt J, 1988, Folstein et al., 1975) Participants were also administered other tests starting in 1997, including: immediate and delayed recall trials of the East Boston Memory Test (EBMT); category fluency test (number of animals named within 60 seconds); delayed-recall trial of a 10-word list; and digit-span backwards.(Brandt J, 1988, Albert et al., 1991) Initial cognitive assessments were completed between 1995-2001. The telephone-based assessment has excellent reliability and validity.(Stampfer et al., 2005)
Study outcomes were composite scores of global, verbal memory, and executive function/attention. Based on means and standard deviations from the first cognitive wave, z-scores were calculated for each test. The global score was created by averaging z-scores of all cognitive tests. Verbal memory was calculated by averaging z-scores of 4 tests: the immediate and delayed recall of the EBMT and 10-word list. The executive function/attention score was created by averaging z-scores of the category fluency and digit span backwards tests.(Hedden et al., 2012) Composite scores were only calculated if the participant had completed all component tests.
2.4 Statistical analysis
Cognitive outcomes were modeled continuously using SAS PROC MIXED (SAS v. 9.3, SAS Institute, USA). We used linear mixed-effects models with person-specific random-effects, where cognitive interview date was the time index. Basic models included time, age (years, continuous), education (RN, BA, or advanced) and the three depression pattern groups, as well as interactions of these terms with time. Multivariable model covariates were selected a priori based on prior literature and ascertained from the questionnaire returned immediately before initial cognitive testing. The multivariable-adjusted model included all terms from the basic model, plus the following covariates and their interactions with time: smoking (current, past, never), alcohol use (grams/day), body mass index (BMI) (kg/m2), and physical-activity (MET hrs/week). Finally, an expanded model added other covariates, including medical comorbidities that may represent either confounders or intermediates. This model included all covariates from the above multivariable model plus: cardiovascular disease (history of myocardial infarction and/or coronary artery bypass grafting); hypertension; dyslipidemia; history of cancer other than non-melanoma skin cancer; respiratory disease (asthma and/or chronic obstructive pulmonary disease); diabetes; and antidepressant use. Covariate data were obtained through self-report.
2.5 Secondary analyses
Because depression has been associated with increased attrition, we conducted a sensitivity analysis to address the potential for informative censoring. This was accomplished by repeating all models adjusting for censoring using stabilized inverse probability censoring weights (IPCW) and comparing to unweighted models. (Hernan et al., 2000, Robins, 1999, Robins et al., 2000) Further information on IPCW is available in the Supplemental Methods. Finally, in a supplementary analysis, we examined logistic regression models estimating the relation of depression pattern to likelihood of being in the worst 10% of change from the first to fourth cognitive interview. An advantage of this approach is to provide odds ratios that may be more interpretable in clinical literature. Logistic models were adjusted for all covariates in the expanded multivariable model, plus baseline composite score and timespan of follow-up; we again conducted sensitivity analyses using IPCW.
3. RESULTS
3.1 Participant Characteristics
Table 1 displays baseline characteristics by depression group. There were 10,723 women in the non-depressed group, 763 in the variable group and 189 in the persistent group. The non-depressed group had lower BMI, higher physical activity, higher alcohol consumption, and lower prevalence of co-morbidities. Use of antidepressants among a small proportion of those classified as non-depressed may reflect presence of indications other than depression (e.g., neuropathic pain, insomnia, etc.), indicate maintenance treatment to sustain euthymic mood after previous depression, or signal that depression was present but not at the severity indicated by MHI-5≤52.
Table 1.
Participant Characteristics by Depression Group
| Characteristic* | No Depression | Variable Depression | Persistent** Depression | P-value | |||
|---|---|---|---|---|---|---|---|
| n=10723 | n=763 | n=189 | |||||
| Age, mean (sd) | 74.2 | (2.4) | 74.1 | (2.3) | 74.1 | (2.3) | 0.57 |
| Race | 0.34 | ||||||
| White, % | 98% | 99% | 99% | ||||
| Black, % | 1% | 0% | 1% | ||||
| Other, % | 1% | 1% | 0% | ||||
| Highest Attained Education | 0.06 | ||||||
| RN, % | 77% | 80% | 84% | ||||
| BA, % | 17% | 15% | 12% | ||||
| MA/Doctoral, % | 7% | 5% | 4% | ||||
| BMI, mean (sd) | 26.0 | (4.8) | 26.1 | (5.4) | 26.9 | (5.6) | 0.02 |
| Physical activity score, mean(sd) | 16.5 | (19.9) | 12.4 | (15.7) | 11.0 | (15.1) | <0.0001 |
| Mediterranean Diet Score, mean (sd) | 4.4 | (1.5) | 4.2 | (1.5) | 4.1 | (1.5) | <0.0001 |
| Hypertension, % | 55% | 61% | 61% | <0.003 | |||
| High Cholesterol, % | 67% | 72% | 77% | <0.0001 | |||
| Smoking Status | 0.003 | ||||||
| Never, % | 47% | 43% | 39% | ||||
| Past, % | 46% | 47% | 48% | ||||
| Current, % | 7% | 9% | 13% | ||||
| Alcohol use, mean (sd) | 4.8 | (9.1) | 4.5 | (9.8) | 3.7 | (8.3) | 0.22 |
| Hormone Use | 0.02 | ||||||
| Never, % | 29% | 26% | 25% | ||||
| Past, % | 30% | 32% | 32% | ||||
| Current, % | 33% | 35% | 29% | ||||
| Multi-vitamin use, % | 63% | 68% | 66% | 0.09 | |||
| Antidepressant use, % | 5% | 17% | 30% | <0.0001 | |||
| Cancer, % | 19% | 23% | 21% | 0.05 | |||
| CVD†, % | 10% | 13% | 16% | <0.0001 | |||
| Diabetes, % | 10% | 13% | 18% | <0.0001 | |||
| Respiratory Disease‡, % | 15% | 20% | 31% | <0.0001 | |||
Information on baseline characteristics was obtained via self-reports by participants on NHS questionnaires
No depression – no severe depressive symptoms reported at any baseline time points as determined by a Mental Health Inventory–5 score >52; Variable depression – severe depressive symptoms at some but not all baseline time points; Persistent depression – severe depressive symptoms reported at all baseline time points
CVD includes myocardial infarction, coronary artery bypass grafting, or stroke
Respiratory illness includes asthma or Chronic Obstructive Pulmonary Disease (COPD)
3.2 Multivariable Models
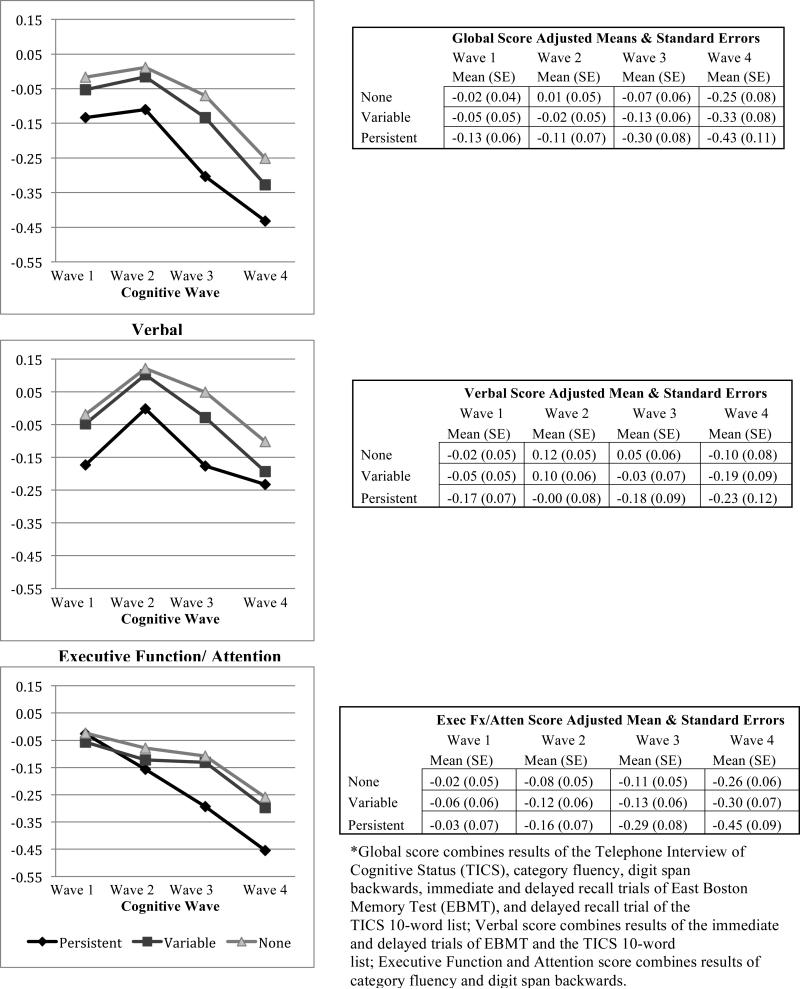
Multivariable-adjusted least-squares mean cognitive scores at baseline and over follow-up (Figure 2) indicated that the persistent group generally had the lowest scores in each of the cognitive domains, and the variable group had scores intermediate to the other categories. However, divergence in the executive function/attention scores over follow-up was notable for the persistent vs. other groups.
Figure 2.
Multivariable Adjusted Mean Cognitive Scores Over Time by Depressive-Symptom Category Global
Basic model results are presented in Model 1 of Table 2. In the main multivariable model (Model 2), adjusted for lifestyle factors, the persistent symptom group had significantly lower global (p=0.001) and verbal-memory scores (p<0.001) cross-sectionally, compared to the non-depressed group. No cross-sectional differences between the persistent and non-depressed groups were observed for the executive-function/attention domain (p=0.52). The variable pattern group showed no statistically significant cross-sectional mean differences compared to non-depressed group. Results were similar after adjusting for co-morbidities and potential intermediates.
Table 2.
Mean Differences in Baseline Cognitive Function and Annualized Rates of Cognitive Decline, by Depressive Symptom Categories
| Depression Category | Global¶ | Verbal¶ | Executive Function & Attention¶ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1* (Age and education adjusted) | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ |
| Cross-Sectional | ||||||||||||
| Persistent Depression | −0.14 | (−0.22, −0.06) | <0.001 | −0.17 | (−0.26, −0.08) | <0.001 | −0.05 | (−0.15, 0.05) | 0.32 | |||
| Variable Depression | −0.04 | (−0.09,−0.004) | 0.03 | −0.03 | (−0.08, 0.01) | 0.16 | −0.06 | (−0.11, −0.01) | 0.03 | |||
| No Depression | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||
| Cognitive Change | ||||||||||||
| Persistent Depression | −0.02 | (−0.04, 0.001) | 0.06 | −0.01 | (−0.03, 0.02) | 0.64 | −0.03 | (−0.05, −0.01) | 0.002 | |||
| Variable Depression | −0.01 | (−0.02, 0.000) | 0.06 | −0.01 | (−0.02, −0.002) | 0.02 | 0.0004 | (−0.01, 0.01) | 0.93 | |||
| No Depression | Ref | Ref | Ref | 3.45; 0.03 | Ref | Ref | Ref | 2.74; 0.06 | Ref | Ref | Ref | 4.73; 0.01 |
| Model 2† (Main model) | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-Sectional | ||||||||||||
| Persistent Depression | −0.13 | (−0.21, −0.05) | 0.001 | −0.16 | (−0.26, −0.07) | <0.001 | −0.03 | (−0.13, 0.07) | 0.52 | |||
| Variable Depression | −0.04 | (−0.08, 0.004) | 0.08 | −0.02 | (−0.07, 0.02) | 0.29 | −0.04 | (−0.09, 0.01) | 0.10 | |||
| No Depression | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||
| Cognitive Change | ||||||||||||
| Persistent Depression | −0.02 | (−0.04, 0.002) | 0.09 | −0.004 | (−0.03, 0.02) | 0.71 | −0.03 | (−0.05, −0.01) | 0.003 | |||
| Variable Depression | −0.01 | (−0.02, 0.002) | 0.11 | −0.01 | (−0.02, −0.001) | 0.03 | 0.0003 | (−0.01, 0.01) | 0.95 | |||
| No Depression | Ref | Ref | Ref | 2.64; 0.07 | Ref | Ref | Ref | 2.28; 0.10 | Ref | Ref | Ref | 4.57; 0.01 |
| Model 3‡ (Including potential intermediates) | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-Sectional | ||||||||||||
| Persistent Depression | −0.10 | (−0.18, −0.02) | 0.01 | −0.13 | (−0.22, −0.04) | 0.005 | −0.01 | (−0.11, 0.09) | 0.89 | |||
| Variable Depression | −0.02 | (−0.06, 0.02) | 0.34 | −0.01 | (−0.05, 0.04) | 0.73 | −0.03 | (−0.08, 0.02) | 0.26 | |||
| No Depression | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||
| Cognitive Change | ||||||||||||
| Persistent Depression | −0.01 | (−0.03, 0.01) | 0.16 | −0.001 | (−0.02, 0.02) | 0.92 | −0.03 | (−0.05, −0.01) | 0.006 | |||
| Variable Depression | −0.01 | (−0.01, 0.004) | 0.26 | −0.01 | (−0.02, 0.001) | 0.09 | 0.002 | (−0.01, 0.01) | 0.70 | |||
| No Depression | Ref | Ref | Ref | 1.54; 0.22 | Ref | Ref | Ref | 1.48; 0.23 | Ref | Ref | Ref | 3.93; 0.02 |
Model 1 (basic model): Age and Education adjusted. Mean interval between first and fourth wave of cognitive tests=5.5 years
Model 2 (basic model + lifestyle factors): Model 1 plus smoking status, alcohol consumption, BMI, physical activity
Model 3 (basic model + lifestyle factors + medical comorbidities + antidepressant use): Model 2 plus vascular risk factors (CVD [myocardial infarction, coronary artery bypass grafting, and/ or stroke], hypertension, dyslipidemia), other major comorbidities (diabetes, respiratory illness [asthma and/or Chronic Obstructive Pulmonary Disease], cancer history other than non-melanoma skin cancer), and antidepressant use
F-statistic is from global test of depression categories by time interaction; All models have 2 df for numerator
Denominator df: (Model 1 global=9231, verbal=9419, exec=9579), (Model 2 global=9224, verbal=9411, exec=9579), (Model 3 global=9217, verbal=9405, exec=9577)
Global score combines results of the Telephone Interview of Cognitive Status (TICS), category fluency, digit span backwards, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; Verbal score combines results of the immediate and delayed trials of EBMT and the TICS 10-word list; Executive Function and Attention score combines results of category fluency and digit span backwards.
When examining cognitive change in the main model, the variable pattern group had 0.01 standard units/year worse decline in verbal memory (95% CI: −0.02 to −0.001) compared to the non-depressed group (p=0.03). By contrast, no significant difference was observed in verbal memory change when comparing the persistent to non-depressed groups (p=0.71). Regarding executive function/attention domain, the opposite was observed: the persistent group had 0.03 standard units/year greater decline (95% CI: −0.05 to −0.01, p=0.003) compared to the non-depressed group; yet, no differences were seen for the variable group (p=0.95). The observed mean difference in cognitive decline in the executive-function/attention domain for the persistently depressed group was comparable to that which would be observed for women 8 years apart in age. No statistically significant differences were observed for cognitive change in the global score comparing the no depression group to either the persistent (p=0.09) or the variable depression groups (p=0.11). Estimates after inclusion of co-morbidities and potential intermediates were generally similar to those from the main model.
3.3 Sensitivity Analyses
When categorizing the sample using only the 1992 and 1996 questionnaires, nearly identical point estimates were observed compared to the main analyses (data not shown in tables). We further considered the possibility that some individuals might be categorized as being in the variable pattern group based on differences in their MHI-5 scores of only a few points. We determined that only 43 participants were assigned to the variable-pattern based on variations of <5 points on MHI-5 scores. In a sensitivity analysis, we excluded these 43 participants and reran the models; results were unchanged.
3.4 IPCW Sensitivity Analyses
Higher attrition was observed for those in the persistent and variable depressive symptom pattern groups compared to the non-depressed group (Supplemental Table 1). To account for possible informative-censoring, we performed sensitivity analyses using IPCW (Table 3). Point-estimates remained similar to those in the main analyses, although results for verbal memory change were attenuated (p=0.07) for the variable group. Findings of steeper decline in executive function/attention among the persistently depressed vs. non-depressed group were similar to main results.
Table 3.
Supplemental Methods: Mean Differences in Annualized Rates of Cognitive Decline by Depressive Symptom Categories using IPCW*
| Depression Category | Global¶ | Verbal¶ | Executive Function & Attention¶ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1** | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ |
| Persistent Depression | −0.02 | (−0.04, 0.001) | 0.07 | −0.01 | (−0.03, 0.02) | 0.63 | −0.03 | (−0.05, −0.01) | 0.004 | |||
| Variable Depression | −0.01 | (−0.02, 0.002) | 0.14 | −0.01 | (−0.02, 0.000) | 0.05 | 0.001 | (−0.01, 0.01) | 0.90 | |||
| No Depression | Ref | Ref | Ref | 2.65; 0.07 | Ref | Ref | Ref | 1.98; 0.14 | Ref | Ref | Ref | 4.30; 0.01 |
| Model 2† | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Persistent Depression | −0.02 | (−0.04, 0.003) | 0.10 | −0.005 | (−0.03, 0.02) | 0.70 | −0.03 | (−0.05, −0.01) | 0.004 | |||
| Variable Depression | −0.01 | (−0.02, 0.004) | 0.23 | −0.01 | (−0.02, 0.001) | 0.07 | 0.001 | (−0.01, 0.01) | 0.80 | |||
| No Depression | Ref | Ref | Ref | 2.00; 0.14 | Ref | Ref | Ref | 1.64; 0.19 | Ref | Ref | Ref | 4.16; 0.02 |
| Model 3‡ | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ | Estimate | 95% CI | p-value | F; p-value§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Persistent Depression | −0.01 | (−0.03, 0.01) | 0.17 | −0.002 | (−0.03, 0.02) | 0.86 | −0.03 | (−0.05, −0.01) | 0.01 | |||
| Variable Depression | −0.004 | (−0.01, 0.01) | 0.43 | −0.01 | (−0.02, 0.003) | 0.16 | 0.003 | (−0.01, 0.01) | 0.56 | |||
| No Depression | Ref | Ref | Ref | 1.19; 0.31 | Ref | Ref | Ref | 1.01; 0.36 | Ref | Ref | Ref | 3.69; 0.03 |
Inverse Probability Censoring Weights (IPCW): Participants weighted by the inverse of their probability of not being censored at the fourth cognitive assessment
Model 1: Age and Education adjusted. Mean interval between first and fourth wave of cognitive tests=5.5 years.
Model 2: Model 1 plus smoking status, alcohol consumption, BMI, physical activity
Model 3 (basic model + lifestyle factors + medical comorbidities + antidepressant use): Model 2 plus vascular risk factors (CVD [myocardial infarction, coronary artery bypass grafting, and/ or stroke], hypertension, dyslipidemia), other major comorbidities (diabetes, respiratory illness [asthma and/or Chronic Obstructive Pulmonary Disease], cancer history other than non-melanoma skin cancer), and antidepressant use
F-statistic is from global test of depression categories by time interaction; All models have 2 df for numerator
Denominator df: (Model 1 global=9558, verbal=9750, exec=9550), (Model 2 global=9563, verbal=9757, exec=9563), (Model 3 global=9552, verbal=9743, exec=9560)
Global score combines results of the Telephone Interview of Cognitive Status (TICS), category fluency, digit span backwards, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; Verbal score combines results of the immediate and delayed trials of EBMT and the TICS 10-word list; Executive Function and Attention score combines results of category fluency and digit span backwards.
3.5 Logistic Models of Worst Cognitive Change
In logistic models of worst cognitive change (Supplemental Table 2), results were comparable to those observed when analyzing change continuously. For example, the persistent-depressive group had increased odds of worst cognitive change compared to the non-depressed group; however, no statistically significant associations were observed. Statistical power was lower in the logistic models than in linear models using continuous cognitive scores.
4. Discussion
In this study of patterns of LLD symptoms and cognition, a pattern of persistent severe symptoms was related to lower scores at all cognitive assessments. However, in analyses of cognitive change over time, the persistent and variable patterns of depressive symptoms related differently to decline in specific cognitive domains. Those persistently reporting severe depressive symptoms at all timepoints had significantly worse declines in executive function/attention, but not other cognitive domains, compared to those never reporting severe depressive symptoms. By contrast, those variably reporting severe depressive symptoms at some but not all timepoints had modest but statistically significant worse subsequent decline in verbal memory – but not in other domains – compared to those never reporting severe symptoms.
These results may have clinical relevance, as domain-specific differences in rates of cognitive change by depression pattern group may signal distinct underlying etiologies. In a 2012 (Hedden et al., 2012) cross-sectional study of 168 cognitively normal adults (65-86 years), amyloid-burden was associated with worse episodic-memory performance, while white-matter-hyperintensities (WMH) were associated most strongly with executive-function deficits. High amyloid-burden is a hallmark of brain pathology in patients with AD, while high WMH burden is more typical in vascular dementia. Vascular dementia frequently presents with early executive dysfunction while deficits in verbal memory are more commonly seen early in AD.(Roman and Royall, 1999, Gomez and White, 2006) The presence of WMH has also been associated with LLD and the vascular depression hypothesis in which a constellation of vascular risk factors predisposes the brain to development of depression.(Taylor et al., 2013) Further, when LLD is accompanied by executive-function deficits, studies indicate especially poor response to antidepressant treatment.(Alexopoulos et al., 2005) Additionally, greater regional burden of WMH has been associated with worse response to antidepressant treatment.(Taylor et al., 2014) It may be that vascular pathology contributes both to a persistent pattern of LLD that is particularly resistant to treatment as well as to the executive dysfunction symptoms seen in vascular dementia. These findings hint towards the possibility that differing courses of depression and domain-specific cognitive declines may signal differences in underlying brain pathologies.
Our results are intriguing, in light of emerging hypotheses regarding the nature of neuropsychiatric symptoms preceding cognitive decline. Verbal (or episodic) memory decline relates closely to amnestic cognitive impairment and is a key predictor of AD.(Chen et al., 2001, Kang et al., 2006) It has been hypothesized that depressive symptoms may be part of the lengthy prodromal phase of AD.(Amieva et al., 2008) Our results suggest that a temporal pattern of variable depressive symptoms, rather than persistently severe symptoms, is related specifically to subsequent episodic memory decline among older adults. If LLD were a risk factor for AD, we would expect that those with persistent severe depressive symptoms would have worse cognitive decline in verbal memory, which is most closely related to AD. Because only variable, not persistent, depressive symptoms were related to episodic memory decline, this may suggest that LLD in such cases represents a prodromal manifestation of underlying illness. Conversely, it is also possible that an unstable course of depressive symptoms is intrinsically more detrimental to verbal memory, and subsequent risk of AD, than a persistent severe course.
Our findings may have clinical implications. Poor executive function is associated with worse performance in activities of daily living.(Johnson et al., 2007) Thus, the significantly worse course of executive function observed among those with persistent depressive symptoms could signal future impairments that may adversely affect independence. Identification of LLD patterns may assist clinicians in monitoring for domain-specific cognitive changes and the associated potential hazards.
Strengths of this study include large sample size, prospective design, and well-validated questionnaire information over many years. Another advantage was the examination of how patterns of depressive symptoms measured at multiple timepoints related to subsequent trajectories within specific cognitive domains. Sensitivity analyses using IPCW to investigate the potential for informative censoring is another strength of our approach.
Study limitations are also important to consider. First, length of cognitive follow-up may have been insufficient to detect differences in the patterns of decline. However, the mean age at cognitive baseline for the cohort was approximately 74 years. Since older age is a known risk factor for cognitive-decline, an average follow-up of almost six years likely captures meaningful trajectories. Additionally, the observed longitudinal pattern of scores illustrates that change is occurring and is evident across the four timepoints. Second, depressive symptoms were categorized based on self-report from screening instruments rather than by gold-standard psychiatric diagnosis. However, use of such instruments provides an opportunity to capture depressive symptoms among those reluctant to seek treatment in a clinical setting. Third, misclassification of depression patterns is possible due to unobserved symptoms between questionnaire intervals. However, such misclassification is likely to be non-differential (i.e., unrelated to the outcome). Although not guaranteed to do so (Dosemeci et al., 1990), such misclassification would likely bias toward the null for effect estimates and, thus, would render our findings more conservative. Further, use of repeated measures to categorize depressive symptoms provides more opportunity to capture gradations of symptoms. Having additional depressive symptom assessments, beyond the 2-3 timepoints included in this study, might have facilitated characterizing patterns with further granularity; however, we used a strictly-defined prospective approach of limiting depression assessments to those occurring prior to the start of cognitive testing. Fourth, although there is little reason to suspect that the relationship between LLD patterns and cognitive decline would differ among demographic groups, generalizability of our results may be a concern, given the small numbers of non-white participants and lack of male participants. Fifth, these highly educated nurses may have higher cognitive reserve than the general population; this could limit generalizability of results. Finally, our study only examined how depression relates to performance on objective cognitive tests; these results do not show how antecedent depression patterns relate to structural or functional changes in the brain.
In conclusion, this study of nearly 12,000 older adults found that antecedent patterns of LLD symptoms were related differentially to subsequent decline in cognitive domains. The presence of persistently severe depressive symptoms was related to subsequent decline in the frontal-executive domain, but not episodic memory. On the other hand, a variable pattern of depressive-symptoms was related to later decline in episodic memory, but not executive function. Our finding of contrasting relations of antecedent patterns of LLD symptoms to declines in different cognitive domains may signal the presence of distinct underlying processes. Future studies should aim to replicate these findings and incorporate neural markers.
Supplementary Material
Key points.
Findings indicate differences in declines in specific cognitive domains by antecedent depressive symptom patterns.
Compared to those without depressive symptoms, those with persistent severe depressive symptoms had greater declines in the executive function/attention domain, while those with variably severe depressive symptoms had greater declines in the verbal memory domain.
Acknowledgments
Research Sponsors: Ms. Gillis was supported by a National Research Service Award, Training Program in Psychiatric Epidemiology T32 MH1711928 and by a National Institute for Occupational Safety and Health (NIOSH) training grant T42 OH008416. Dr. Okereke was supported by a Harvard Medical School Eleanor and Miles Shore Fellowship. This work was supported by US National Institutes of Health (NIH) grants [R01 MH096776, P01 CA087969, UM1 CA186107]. The funding sources were not involved in the data collection, data analysis, manuscript writing or publication.
REFERENCES
- ALBERT M, SMITH LA, SCHERR PA, TAYLOR JO, EVANS DA, FUNKENSTEIN HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57:167–78. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, KIOSSES DN, HEO M, MURPHY CF, SHANMUGHAM B, GUNNING-DIXON F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–10. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, YOUNG RC, MEYERS BS. Geriatric depression: age of onset and dementia. Biol Psychiatry. 1993;34:141–5. doi: 10.1016/0006-3223(93)90383-o. [DOI] [PubMed] [Google Scholar]
- AMIEVA H, LE GOFF M, MILLET X, ORGOGOZO JM, PERES K, BARBERGER-GATEAU P, JACQMINGADDA H, DARTIGUES JF. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–8. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- BERWICK DM, MURPHY JM, GOLDMAN PA, WARE JE, JR., BARSKY AJ, WEINSTEIN MC. Performance of a five-item mental health screening test. Med Care. 1991;29:169–76. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- BRANDT J, S. M, FOLSTEIN M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- BULTMANN U, RUGULIES R, LUND T, CHRISTENSEN KB, LABRIOLA M, BURR H. Depressive symptoms and the risk of long-term sickness absence: a prospective study among 4747 employees in Denmark. Soc Psychiatry Psychiatr Epidemiol. 2006;41:875–80. doi: 10.1007/s00127-006-0110-y. [DOI] [PubMed] [Google Scholar]
- CHEN P, RATCLIFF G, BELLE SH, CAULEY JA, DEKOSKY ST, GANGULI M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853–8. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- CUIJPERS P, SMITS N, DONKER T, TEN HAVE M, DE GRAAF R. Screening for mood and anxiety disorders with the five-item, the three-item, and the two-item Mental Health Inventory. Psychiatry Res. 2009;168:250–5. doi: 10.1016/j.psychres.2008.05.012. [DOI] [PubMed] [Google Scholar]
- DOSEMECI M, WACHOLDER S, LUBIN JH. Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol. 1990;132:746–8. doi: 10.1093/oxfordjournals.aje.a115716. [DOI] [PubMed] [Google Scholar]
- DOTSON VM, BEYDOUN MA, ZONDERMAN AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLSTEIN MF, FOLSTEIN SE, MCHUGH PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN B, HEISEL M, DELAVAN R. Validity of the SF-36 five-item Mental Health Index for major depression in functionally impaired, community-dwelling elderly patients. J Am Geriatr Soc. 2005;53:1978–85. doi: 10.1111/j.1532-5415.2005.00469.x. [DOI] [PubMed] [Google Scholar]
- GOMEZ RG, WHITE DA. Using verbal fluency to detect very mild dementia of the Alzheimer type. Arch Clin Neuropsychol. 2006;21:771–5. doi: 10.1016/j.acn.2006.06.012. [DOI] [PubMed] [Google Scholar]
- GRAZIANE JA, BEER JC, SNITZ BE, CHANG CC, GANGULI M. Dual Trajectories of Depression and Cognition: A Longitudinal Population-Based Study. Am J Geriatr Psychiatry. 2016;24:364–73. doi: 10.1016/j.jagp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDDEN T, MORMINO EC, AMARIGLIO RE, YOUNGER AP, SCHULTZ AP, BECKER JA, BUCKNER RL, JOHNSON KA, SPERLING RA, RENTZ DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32:16233–42. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNAN MA, BRUMBACK B, ROBINS JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- HOLMES WC. A short, psychiatric, case-finding measure for HIV seropositive outpatients: performance characteristics of the 5-item mental health subscale of the SF-20 in a male, seropositive sample. Med Care. 1998;36:237–43. doi: 10.1097/00005650-199802000-00012. [DOI] [PubMed] [Google Scholar]
- JOHNSON JK, LUI LY, YAFFE K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–41. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG JH, COOK N, MANSON J, BURING JE, GRODSTEIN F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–8. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- KOHLER S, VAN BOXTEL MP, VAN OS J, THOMAS AJ, O'BRIEN JT, JOLLES J, VERHEY FR, ALLARDYCE J. Depressive symptoms and cognitive decline in community-dwelling older adults. J Am Geriatr Soc. 2010;58:873–9. doi: 10.1111/j.1532-5415.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- OKEREKE OI, GRODSTEIN F. Phobic anxiety and cognitive performance over 4 years among community-dwelling older women in the Nurses’ Health Study. Am J Geriatr Psychiatry. 2013;21:1125–34. doi: 10.1016/j.jagp.2013.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIN JT, KATZ IR, MEYERS BS, SCHNEIDER LS, LEBOWITZ BD. Provisional diagnostic criteria for depression of Alzheimer disease: rationale and background. Am J Geriatr Psychiatry. 2002;10:129–41. [PubMed] [Google Scholar]
- PATERNITI S, VERDIER-TAILLEFER MH, DUFOUIL C, ALPEROVITCH A. Depressive symptoms and cognitive decline in elderly people. Longitudinal study. Br J Psychiatry. 2002;181:406–10. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- ROBINS J. Association, Causation, and Marginal Structural Models. Synthese. 1999;121:151–79. [Google Scholar]
- ROBINS JM, HERNAN MA, BRUMBACK B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- ROMAN GC, ROYALL DR. Executive control function: a rational basis for the diagnosis of vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S69–80. doi: 10.1097/00002093-199912003-00012. [DOI] [PubMed] [Google Scholar]
- RUMPF HJ, MEYER C, HAPKE U, JOHN U. Screening for mental health: validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry Res. 2001;105:243–53. doi: 10.1016/s0165-1781(01)00329-8. [DOI] [PubMed] [Google Scholar]
- SINGH-MANOUX A, AKBARALY TN, MARMOT M, MELCHIOR M, ANKRI J, SABIA S, FERRIE JE. Persistent depressive symptoms and cognitive function in late midlife: the Whitehall II study. J Clin Psychiatry. 2010;71:1379–85. doi: 10.4088/JCP.09m05349gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMPFER MJ, KANG JH, CHEN J, CHERRY R, GRODSTEIN F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–53. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- STEFFENS DC, MCQUOID DR, PAYNE ME, POTTER GG. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2011;19:4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEFFENS DC, PLASSMAN BL, HELMS MJ, WELSH-BOHMER KA, SAUNDERS AM, BREITNER JC. A twin study of late-onset depression and apolipoprotein E epsilon 4 as risk factors for Alzheimer's disease. Biol Psychiatry. 1997;41:851–6. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- TAYLOR WD, AIZENSTEIN HJ, ALEXOPOULOS GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR WD, KUDRA K, ZHAO Z, STEFFENS DC, MACFALL JR. Cingulum bundle white matter lesions influence antidepressant response in late-life depression: a pilot study. J Affect Disord. 2014;162:8–11. doi: 10.1016/j.jad.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DUIJN CM, CLAYTON DG, CHANDRA V, FRATIGLIONI L, GRAVES AB, HEYMAN A, JORM AF, KOKMEN E, KONDO K, MORTIMER JA, ROCCA WA, SHALAT SL, SOININEN H, HOFMAN A, et al. Interaction between genetic and environmental risk factors for Alzheimer's disease: a reanalysis of case-control studies. Genet Epidemiol. 1994;11:539–51. doi: 10.1002/gepi.1370110609. [DOI] [PubMed] [Google Scholar]
- WARE JE, JR., SHERBOURNE CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- ZUBENKO GS, ZUBENKO WN, MCPHERSON S, SPOOR E, MARIN DB, FARLOW MR, SMITH GE, GEDA YE, CUMMINGS JL, PETERSEN RC, SUNDERLAND T. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer's disease. Am J Psychiatry. 2003;160:857–66. doi: 10.1176/appi.ajp.160.5.857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.