Abstract
Hostility is a risk factor for cardiovascular events. When challenged individuals high on hostility exhibit a hyper-reactive psychophysiological response to stressors, thereby increasing risk for developing cardiovascular disease. However, low resting heart rate (HR) is associated with physical aggression and hostility in children, adolescents, and adults. Based on a community sample of 296 men (mean age=32.0, standard deviation = .9), we address: 1) whether aggression/hostility relates to physical health through relationships with cardiovascular levels at rest and in response to stressors and 2) determine how relations between aggression and health are altered by including psychophysiological indices in statistical models. The Cook-Medley cynical/hostile attitudes and the Buss-Perry physical aggression and hostility measures assessed aggression. Health was assessed as systolic blood pressure (SBP), report of medical conditions, and a metabolic composite. Reactivity to stressors was assessed with HR, SBP, and diastolic blood pressure. The relationship between aggression and health was significantly strengthened when HR level and reactivity were included in models. Aggression was negatively related to both resting HR and reactivity. High resting HR and reactivity were, however, positively related to poor health. The relationship between aggression and HR and reactivity suppressed an overall relationship between high aggression/hostility and poor health. Socioeconomic status (SES), race, health behaviors, and medications were examined as covariates. At early midlife, low HR among aggressive and hostile individuals is related to less health risk. Aggression and hostility have a deleterious influence on health, but primarily among individuals with higher HR and possibly greater cardiovascular reactivity.
Keywords: Hostility, aggression, HR, blood pressure, reactivity
Individual differences in resting HR and cardiovascular responses to laboratory stress exhibit divergent associations with various physical and behavioral health outcomes. On the one hand, high resting HR is a robust risk factor for cardiovascular disease and mortality (Menown et al., 2013). On the other hand, low resting HR is a risk factor for aggression and criminal offending (Latvala, Kuja-Halkola, Almqvist, Larsson, & Lichtenstein, 2015; Ortiz & Raine, 2004; Raine, Fung, Portnoy, Choy, & Spring, 2014). Independent of resting HR, heightened HR during stress is associated with positive adjustment in children (Boyce et al., 1995; Boyce & Ellis, 2005), less non-cardiac disease and depression (Phillips, 2011; Phillips, Ginty, & Hughes, 2013; Phillips, Hunt, Der, & Carroll, 2011) and better cognitive performance (Ginty, Phillips, Der, Deary, & Carroll, 2011). However, heightened HR during stress has also been related to pre-clinical signs of cardiovascular disease (Kaplan, Pettersson, Manuck, & Olsson, 1991; Manuck, Kaplan, Adams, & Clarkson, 1989; Manuck, Kaplan, & Clarkson, 1983). Taken together, these results suggest that global conclusions regarding how psychological and physical health relates to resting HR and HR responses during stress cannot be justified. More nuanced conclusions seem likely if we could understand how aggression and hostility relate to both health and resting HR and psychophysiological reactivity to stress.
Hostility, anger, and aggression are related conceptually and empirically to one another. Hostility is the cognitive component and entails cynical attitudes and mistrust of others. Anger is the emotional or affective component and usually involves physiological activation; it can trigger aggression. Aggression, both physical and verbal, involves hurting or harming others, and represents the behavioral component. Hostile attitudes toward others relate positively to cardiovascular risk and overall mortality e.g., (Chida & Steptoe, 2009; Iribarren et al., 2005; Iribarren et al., 2000; Klabbers, Bosma, van den Akker, Kempen, & van Eijk, 2013; Siegler, Peterson, Barefoot, & Williams, 1992; Wong, Sin, & Whooley, 2014). Furthermore, a meta-analysis reports that individuals who are aggressive, Type A, or hostile exhibit greater blood pressure and HR responses to stressful interpersonal encounters than their counterparts (Chida & Hamer, 2008; Suarez, Kuhn, Schanberg, Williams, & Zimmermann, 1998; Suarez & Williams, 1989, 1990). Larger blood pressure reactions to stressful challenges have also been related to subsequent cardiovascular disease (Jennings et al., 2004; Matthews et al., 2004; Matthews, Zhu, Tucker, & Whooley, 2006; Treiber et al., 2003). General arousal concepts, which posit a constellation of heighted cardiovascular levels and responsivity, including both HR and blood pressure, cannot explain the divergence in relationships between hostility/aggression and these cardiovascular measures, see critiques of general physiological arousal (Lacey & Lacey, 1974; Lacey, 1967; Venables, 1984). HR and blood pressure levels and their reactivity to stress may not be indexing a general over or under arousal state. If not, then we may expect relationships to health and behavior to vary between measures and individuals. This is particularly evident, given that HR reactivity is not consistently related cardiovascular risk, e.g., (Jennings et al., 2004). The puzzle is how poor health can be related to both high HR and aggression/hostility while aggression/hostility is also related to low HR.
Current Study
This puzzle may be clarified by an empirical examination of health, HR, and hostility/aggression. We did so with psychophysiological measures of HR and blood pressure, self-report of hostile attitudes and physical aggression, self-reported health conditions, and metabolic syndrome indices in adult men who were participating in a longitudinal study, the Pittsburgh Youth Study. This study was based on a public- school based sampling with selection designed to yield about half of the children in the top third of the distribution of risk for anti-social behavior (Fite, Raine, Stouthamer-Loeber, Loeber, & Pardini, 2010; Loeber et al., 2012; Loeber, Stouthamer-Loeber, & Farrington, 2008).
Our approach was to relate measures of aggression/hostility to health within a structural equation model (SEM) that included paths through HR and cardiovascular reactivity. Health in this relatively young sample was modeled as a combination of blood pressure, disease reporting, and metabolic indices. The aggression/hostility to health path was based on the known relationship of the Cook-Medley scale to poor health outcomes, though less is known about the health risk of aggressive behavior (Chida & Hamer, 2008; Klabbers et al., 2013; Siegler et al., 1992; Wong et al., 2014).
The model conceptualizes low HR as an expression of individual differences in aggression/hostility. HR is further included because of literature relating high HR to mortality (Menown et al., 2013). From the literature in behavioral medicine, e.g, (Chida & Steptoe, 2009) one might infer that aggression/hostility would be associated with high heart rate, i.e. a high activation state, and hence risk for mortality. Prior work, however, has consistently shown relations between high aggression and low HR level (Latvala et al., 2015; Lorber, 2004; Portnoy & Farrington, 2015). Psychologically, low HR in hostile/aggressive individuals has been conceptualized as indicating states which would foster greater expression of hostility aggression (Portnoy et al., 2014). An absence of fear/anxiety would eliminate one impediment to the expression of hostility/aggression. In the absence of fear/anxiety, HR might be expected to be relatively low. Alternatively, or in addition, a state of low activation indexed by HR might induce a drive for greater sensation seeking expressed as aggression. These interpretations also relate to early psychophysiological work suggesting low activation in sociopathic and psychopathic individuals, see (Zahn, 1986). Note that these interpretations might suggest that low heart rate initiates the aggressive/hostile tendencies. Our basic model, however, expresses low HR as a specific expression of aggression/hostility rather than an index of a general activation state preceding hostility/aggression. An alternative model can be created in which HR initiates aggressive/hostile tendencies, but direction of causation cannot be determined with the current cross-sectional data.
Reactivity was included in the model because of reports relating cardiovascular reactivity to hostility (Chida & Steptoe, 2009) and to cardiovascular disease (Matthews et al., 2004; Treiber et al., 2003). The rationale for reactivity relating to disease has been previously developed, e.g. (Krantz & Manuck, 1984); disease risk arises from either repeated or greater magnitude cardiovascular responses to stress that induce adverse mechanical and metabolic influences on the cardiovascular system.
In sum, we applied a SEM that hypothesized latent constructs representing aggression/hostility, health, and psychophysiological reactivity. A path from aggression/hostility to health was hypothesized as were indirect paths from aggression/hostility through HR and psychophysiological reactivity to health. The aim was to fit this model and examine the how direct and indirect paths interacted.
Method
Participants
We report on 296 male adult participants from the longitudinal Pittsburgh Youth Study who were recruited into the present study. These individuals agreed to the psychophysiological study and were not taking any medication influencing HR, e.g. beta-blockers, although other medications treating blood pressure were permitted. Men in the Pittsburgh Youth Study were contacted to participate in the current study focused on risk for cardiovascular disease in adulthood. Of the remaining 395 men available for recruitment, 312 participated (79%). Among those eligible but who did not participate, 27% (n=22) declined participation, 23% (n=19) failed to respond to contact or missed appointments, and 51% (n=42) could not be located. Physiological measures were unavailable due to refusal or technical issues in 5% of the participating men (16 of 312). Table 1 presents the characteristics of the remaining men (mean age 33, range: 30–41years). Note that due to the missing data for some variables, e.g. due to difficulty with blood draws, the sample size varies slightly between different variables. University of Pittsburgh Institutional Review Board approved all procedures.
Table 1.
Characteristics of the sample.
| Variable | Valid N | Mean or percent | Std.Dev. |
|---|---|---|---|
| Race (percent African American) | 296 | 56 | |
| Body Mass Index (kg/m2) | 296 | 29.4 | 7.1 |
| Socioeconomic Status (Hollingshead index) | 296 | 31.1 | 14.4 |
| Resting HR (bpm, beats per minute) | 296 | 68.9 | 11.1 |
| Resting SBP (mmHg, millimeters mercury) | 296 | 124.8 | 12.7 |
| Change in HR (mean over tasks, bpm) | 295 | 6.4 | 3.9 |
| Change in SBP (mean over tasks, mmHg) | 290 | 6.4 | 7.7 |
| Physical Aggression (Buss Perry) | 296 | 20.5 | 6.3 |
| Hostility (Buss Perry) | 296 | 14.2 | 6.0 |
| Cynicism (Cook-Medley) | 296 | 6.9 | 3.1 |
| High density lipoprotein (mg/dl) | 295 | 46.5 | 12.7 |
| Triglyceride (mg/dl) | 294 | 129.1 | 94.9 |
| Glucose (mg/dl) | 295 | 95.7 | 28.9 |
| Waist (cm) | 295 | 94.8 | 16.5 |
| Medical Burden (count of conditions) | 296 | 1.2 | 1.2 |
| Adult Physical Activity (Paffenbarger, kilocalorie) | 296 | 1546 | 1945 |
| Adult Alcoholic Drinks per week | 296 | 5.5 | 7.8 |
| Adult Cigarette Use (percentage non-smoker) | 296 | 44 | |
| Adult Prescription Medication Use (percent) | 296 | 6 |
Procedure
Examinations required multiple days with a portion of one day devoted to psychophysiological data collection. Consent and questionnaire administration occurred on the same day as the psychophysiological examination. Participants were required to refrain from cigarettes, caffeine-containing drinks, and heavy exercise for 3 hours prior to the testing and refrain from alcohol or street drugs for 24 hours prior to participation. Participants were uniformly seated in a comfortable chair throughout the psychophysiological testing. A 10 minute resting period preceded the administration of more active tasks. A mirror star tracing task or a mental arithmetic task (see (Matthews, Woodall, & Stoney, 1990) randomly chosen, initiated the session and was followed by a 5 minute inter-task rest and the task not selected to be first. After an additional rest the participant prepared for a speech about a personally annoying event for 3 minutes and then gave the speech for 4 minutes. The session concluded with a post-task resting baseline of 10 minutes. Ratings of task difficulty and engagement followed each task.
Measures
Aggression and Hostility
Aggression was measured by the 9-item physical aggression subscale of the Buss-Perry Aggression scale (Buss & Perry, 1992). Sample items are “Given enough provocation, I may hit another person. I have threatened people I know”. Hostility was measured with the 8-item subscale of the Buss-Perry Aggression scale. Sample items are “Other people always seem to get the breaks. I am suspicious of overly friendly strangers”. Items were rated on a 5-point scale from extremely uncharacteristic to extremely characteristic. Internal consistency was high, for physical aggression, alpha = .85, for hostility, alpha=.77.
The Cook-Medley subscale assessing cynical hostility was administered (Barefoot, Dodge, Peterson, Dahlstrom, & Williams, 1989; Cook & Medley, 1954; Costa, Zonderman, McCrae, & Williams, 1986). It contains 13 items that are rated as true or false. Sample items are “It is safer to trust nobody. I think most people would lie to get ahead.” Internal consistency for this scale version has been reported as alpha=.74 (http://www.cmu.edu/common-cold-project/measures-by-study/psychological-and-social-constructs/personality-measures/hostility.html).
Psychophysiological Measures
Electrocardiogram signals were collected continually during the experimental session. A modified Lead 2 electrocardiogram was employed and attachment and collection follow procedures stipulated in the guidelines for this measure (Jennings et al., 1981). This and the signal from a respiratory belt were transduced by Biopac System (Goleta, CA) modules (ECG and RSP modules of 100C series). All signals were digitized with a 16 bit analog to digital converter and stored for analysis by Mindware Technology (Gahanna, OH) software.
Measures of mean HR as well as low (.04 to .12 Hz) and high frequency (HF, .12–.4 Hz) HR variability were derived using the HR variability module (Mindware, HRV 3.0.21).
Blood pressure was available using a CARESCAPE Dinamap V100 Vital Signs Monitor (GE Medical Systems Information Technologies, Inc.) with a standard occluding cuff place on the participant’s nondominant arm. The automated blood pressure monitor collected blood pressures every two minutes during the rest and task periods. Participants were seated throughout data collection.
HR and blood pressure measures were averaged within each rest or task period. Reactivity measures were created by subtracting mean resting values from the task period values. The resting values from the 5 minute pre-task period and the 10 minute post-task period were highly correlated for all variables, r’s ranged between .80 and .95. Due to this a baseline value averaged across these periods was used. Similarly for the tasks, reasonably high correlation was observed among the tasks for all measures (r’s ranged from .73 to .94). None of the tasks showed differing patterns of correlation with measures from the other tasks. Given this, responses for each variable within each task were standardized and these standardized scores were averaged across tasks. This resulted in overall reactivity scores for each variable. Simple difference scores were used as correlation between baseline and change scores were low and variable. Table 1 includes the mean values for the overall reactivity scores; Supplementary Table 1 presents individual task reactivity values and includes diastolic blood pressure and pre-ejection period variables that are not detailed further presently.
Participant Measures/Covariates
A number of descriptors of the participants and their health behaviors were assessed and examined as measures that might influence health outcomes and relationships with HR and hostility/aggression. Body mass index (BMI) was assessed as weight in kilograms divided by the square of height in meters. Race was self-reported and categorized into Caucasian or African American. Smoking was self-reported and categorized into never, not in last year, less than 10 cigarettes and greater than 10 cigarettes per day in the last year. Alcohol intake was self-reported and scaled as average amount of alcohol per week. Physical activity in estimated kilocalories was derived from the Paffenbarger questionnaire (Paffenbarger et al., 1993). Socioeconomic status (SES) was derived using the Hollingshead index (Hollingshead, 1975), which incorporates educational attainment and occupational status.
Health Outcomes
There are three major health outcomes: metabolic syndrome; resting BP; and total medical burden. Metabolic factors were assessed via blood draw. Serum glucose was determined by electroimmunoassay. The lab coefficient of variation (CV) is 2.1%. Insulin was measured using an RIA procedure, with a lab CV of 2.6%. Total cholesterol, HDL-C and triglycerides were determined by conventional enzymatic procedures. The lab CV between runs are 1.3% and 2.1% respectively. Measures composing the metabolic syndrome were derived from the ATP-III standards (Grundy et al., 2005). Due to the age of our participants, the binary metabolic syndrome score was not used but each of the components was measured and converted to a z score based on our sample. Components were then combined using unit weighting (Jennings et al., 2013). Metabolic risk factors include resting SBP, abdominal obesity (waist circumference), triglycerides, HDL-C (weighted-1 when combined), and fasting glucose. SBP was not included in the current composite due to its separate use as an overall health indicator. Similar metabolic scores have been shown longitudinally to relate to cardiovascular disease events in large epidemiological studies, e.g. (Merkin, Karlamangla, Elashoff, Grogan, & Seeman, 2015).
SBP averaged over pre and post task rest periods period was used as an index of the typical blood pressure of the individual.
Medical burden was determined from a clinical interview covering common medical conditions: cardiovascular, asthma, epilepsy, arthritis, diabetes, endocrine, digestive, pulmonary, and neurologic. A value of 1 was assigned if the participant reported that a nurse or doctor ever told them they had the condition in question. Burden was determined by a simple sum of the conditions reported. One or more conditions was reported by 59% of the participants; no more than 4 conditions were reported by any participant (2% of the sample reported 4 conditions). For analytic purposes this measure was dichotomized in no reported condition or any reported condition.
Analyses
Pearson product-moment correlations were used to examine bivariate associations between aggression and health variables. The multivariate paths between aggression, heart rate, reactivity, and health indices were then modeled using structural equations. This permitted us to concurrently examine the relationships of HR and reactivity and examine the aggression/health relationship with and without these variables in the model. Mediation and moderation analyses could then be done in the context of the SEM.
Results
Participant Characteristics
The characteristics of the participants are shown in Table 1. These characteristics are reasonably typical for the age of the participants (mean age 32, standard deviation .9). The sample is though over half African American, somewhat overweight, and reporting a substantial amount of cigarette use.
Bivariate Relationships of Psychophysiological Measures and Hostility, Aggression, and Health Outcomes
Bivariate correlations confirmed expected relationships between HR and aggression/hostility measures as well as relationships between high heart rate and high reactivity and health outcomes. Table S2 provides a complete set of correlations. Key correlations were in agreement with literature relating lower HR to greater physical aggression (r=−.17) and hostility (r=−.17). As illustrated in Figure 1 higher HR was significantly related to higher scores on the metabolic composite (r=.43). Greater SBP reactivity was also related to higher scores on the metabolic composite (r=.17). These results established an empirical basis for examining how individual differences in aggression were related to health potentially via the psychophysiological indices.
Figure 1.
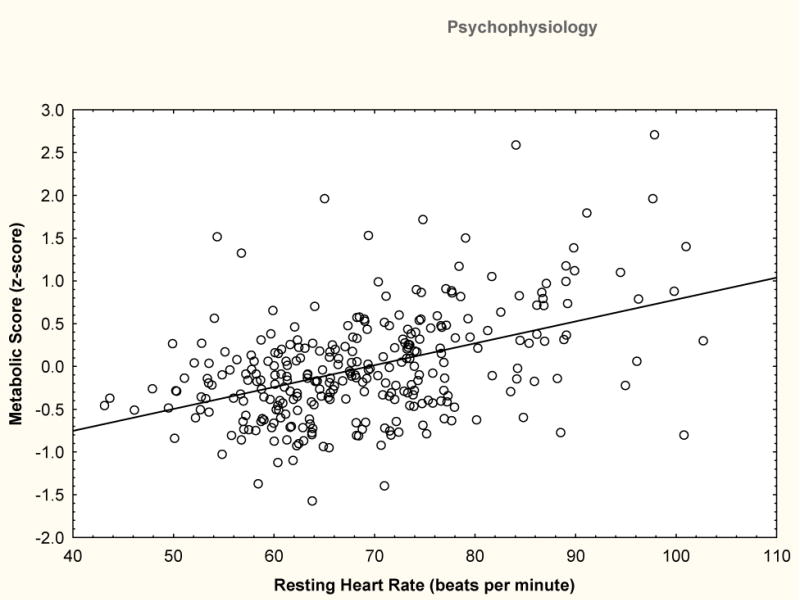
Cardiometabolic score shown as a function of mean resting HR. Cardiometabolic score is a continuous measure formed from the equally weighted combination of factors in the Metabolic Syndrome (waist circumference, glucose, high density lipoprotein (negative weight), triglycerides) with the exception of SBP.
Structural Equation Modelling
SEM was used to examine our hypotheses as the paths to poor health through heart rate and reactivity could be concurrently examined. Three latent factors were created: Health (SBP level, presence or absence of medical complaints, and metabolic factors—a summary score of equally weighted waist circumference, high density lipoprotein (negative weight), and glucose), Aggression (Buss-Perry physical aggression, Buss-Perry hostility, and Cook-Medley cynicism), and Reactivity (SBP change from baseline, diastolic blood pressure change from baseline, and HR change from baseline). We examined as covariates race (African American or Caucasian), socioeconomic status (SES, Hollingshead index), physical activity (Paffenbarger), alcohol use (drinks per week), medication use, and smoking (cigarette use as none, past, less than 10 in past week, more than 10 in past week). Body mass index was not examined as it was represented as waist circumference in the dependent Health latent outcome variable. Only smoking correlated with the Health outcome variable so it was retained in modeling. The Hollingshead socioeconomic status variable was also retained given the significant variation of this within the current sample. Supplementary table S2 presents the correlations upon which the SEM was based including the correlations of potential covariates as well as those used in the model.
Confirmatory factor analyses tested the measurement model to confirm that the index variables appropriately loaded on the latent variables. For each of the latent variables the fit was excellent confirming the measurement model (RMSEA<.001, CFI=1.00). Indicators for the latent Health variable were chosen to include measured indicators of poor health at this relatively early age: blood pressure and glucose and lipid regulation, as well as self-report of medical conditions. The factor analytic loadings on the Health latent variable were .80 and .81 for SBP and the metabolic index and .51 for the self-report of medical conditions. Indicators for Aggression included reports of both physical aggressions as well as hostile and cynical attitudes. Loadings on the Aggression variable were .79 for Buss Perry physical aggression, .74 for Cook Medley cynicism, and .83 for Buss Perry hostility. Indicators for the Reactivity latent variable were systolic and diastolic blood pressure change as well as heart rate change. Loadings on the Reactivity variable were .88 for SBP reactivity, .86 for diastolic blood pressure reactivity, and .66 for HR reactivity.
As shown in Figure 2, Aggression and Reactivity as well as HR level were diagrammed as paths to Health. We examined the strength of these paths as well as in this model and also in models with HR and Reactivity deleted. Following this we tested mediation of the relationship between Aggression and Health by either HR level or Reactivity.
Figure 2.
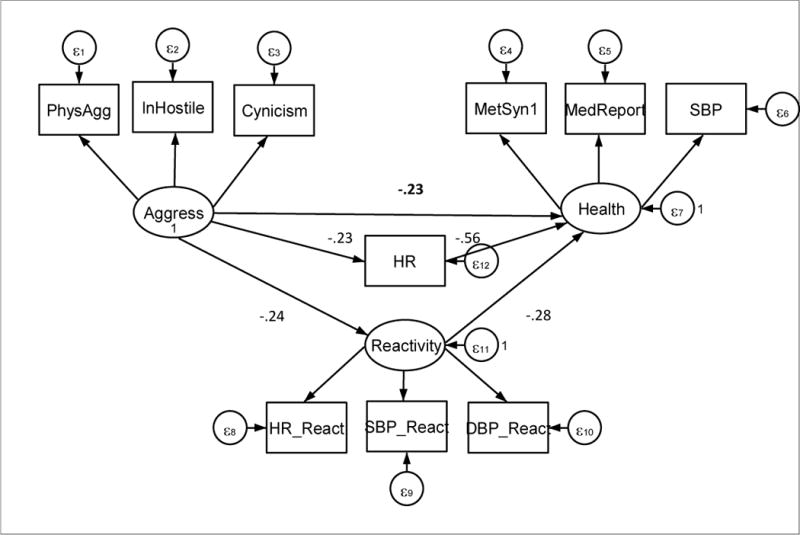
SEM model. Latent factors are Aggression, Health, and Reactivity. Ln Hostility indicates that the natural log of the Buss Perry hostility measure was used as an indicator variable, PhysAgg is the physical aggression scale of the Buss-Perry, and Cynicism is the Cook-Medley Hostility scale. MetSyn1 indicates the linear combination of metabolic syndrome variables excluding SBP, systolic blood pressure. DBP indicates diastolic blood pressure. MedReport is the present or absence of a reported medication condition(s). ε indicates random error and is subscripted to indicate association with different terms of the model.
The model in Figure 2 was fit using the basic correlation coefficients to yield standardized estimates of path strength. Stata 14 (College Station, Texas) was used to estimate the model. Socioeconomic status (Hollingshead) as well as smoking were included in the model as covariates influencing Health although these are not diagrammed in Figure 1 for simplicity of presentation. Missing data were handled through full information maximum likelihood estimation (Schafer & Graham, 2002). The result was an acceptable fit (RMSEA=.06 and CFI=.919) in accordance with criteria set out by Hu and Bentler (1999). The chi square comparing the model to the saturated model was 100 and the Bayesian information criterion was 14902. The model was re-run with HR modeled as initiating Aggression rather than as a function of Aggression. The result was comparable but with a less acceptable fit (RMSEA=.08 and CFI=.85); chi square =142 with a Bayesian information criterion of 14273.
Examining the relationships in our basic model (Figure 2), there is a significant direct path between Aggression and Health (−.23, se=.08, z=−2.75, p=.001). This supports prior work relating greater aggression to poor health. Both HR (−.56, se=.06, z=9.20, p<.001) and Reactivity (−.28, se=.08, z=3.76, p<.01) are inversely related to Health: high heart rate is associated with poor health as is high reactivity. Aggression is not related to either high HR or high Reactivity, but to low HR (−.23, se=.08, z= −3.55, p> 0.001) and low Reactivity (−.24, se=.07. z=−3.48, p<0.001).
Aggression was no longer significantly related to Health when HR and Reactivity were removed from the model. With both removed from the model Aggression was related to Health with a coefficient of −.06, se=.09, z=−.65, ns. Removal of HR alone from the model yielded a coefficient relating Aggression to Health of −.15, se=.09, z=−1.63, p=.10. Removal of Reactivity alone from the model yielding a coefficient relating Aggression to Health of −.16, se=.08, z=−1.95, p=.05. For all of these reduced models RMSEA was less than or equal to .07 and CFI was greater or equal to .91.
Aggression is related to Health more strongly when the relationships with HR and Reactivity are entered into the model. The basis for this can be seen in the overall model. Both HR and Reactivity are negatively related to Health; higher HR and higher Reactivity are related to poorer Health. Aggression, however, is related to lower HR and lower Reactivity. The Sobel test for mediation (Sobel, 1982) can test the strengthening of the Aggression to Health path by the indirect paths through HR and Reactivity. Taken together, the indirect paths to Health through HR and Reactivity are significant (−.248, se=.070, z=3.56, p<.001). Testing for mediation shows that the indirect path significantly accounts for the relationship between Aggression and Health for both HR (Sobel test=3.00, p=.003) and Reactivity (Sobel test=2.35, p=.02).
The strengthening of the relationship between Aggression and Health through the indirect paths suggested that individual differences in HR or Reactivity might moderate the relationship of Aggression and Health. This was examined by separating HR and Reactivity into terciles and performing a group based SEM on each. The degree of measurement invariance between the index and latent variables was examined for the tercile groups using the Lagrange multiplier test. In addition models were fit with and without measurement invariance. These tests suggested reasonable measurement invariance. Some variability in indicator test variance was indicated, however, across groups. Overall fit indices for measurement models across groups were generally acceptable with and without constraining measurement parameters (RMSEA<=.06, CFI>= .93). Heart rate groups showed marginal fit though without measurement invariance for the Health factor (RMSEA=.06, CFI=.693).
Moderation was evident for HR level; only the high HR tercile showed the anticipated relationship between high heart rate and Poor Health (see Table 2). No significant relationship was present for those with lower heart rate. The model fit for this analysis was acceptable (RMSEA=.052, CFI=.910). Within each tercile significant mediation of the Aggression-Health relationship failed to be significant by the Sobel test. The overall moderation effect for HR tercile is illustrated in Figure 3. The figure illustrates the relationship by plotting Aggression against Health separately for the first tertile (low) and the third tercile (high) of HR. As shown low HR individuals fail to show a correlations between Aggression and Health (r=.02, ns) while high HR individuals do show a correlation (r=−.21, p=.04).
Table 2.
Results of mediation analyses for HR level and Reactivity.
| Aggress to Poor Health | Coefficient | Standard Error | Z | P |
|---|---|---|---|---|
| HR Tercile 1 | .113 | .161 | .70 | Ns |
| HR Tercile 2 | .220 | .139 | 1.56 | Ns |
| HR Tercile 3 | .439 | .139 | 3.15 | .002 |
| Reactivity Tercile 1 | −.006 | .288 | −.02 | Ns |
| Reactivity Tercile 2 | .125 | .158 | .79 | Ns |
| Reactivity Tercile 3 | .264 | .216 | .216 | Ns |
Ns is not statistically significant
Figure 3.
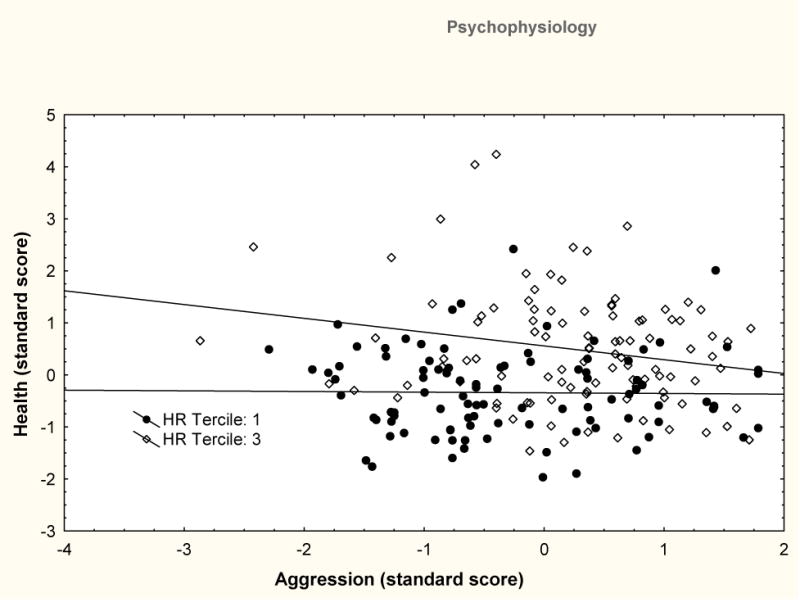
Scatterdiagram of Aggression and Health with individuals from the lower tercile of HR level (filled squares) and the higher tercile of HR level (open triangles) shown separately with a linear fit to their data. No relationship is evident for low HR individuals (r=.02). High HR individuals show a correlation of r=−.21.
Reactivity tercile did not appear to moderate the Aggression-Health relationship. The model fit for this was, however, poor (RMSEA=.18) so the observed coefficients are questionable (see Table 2). Correlations between observed reactivity scores and observed health indices were, however, computed within each tercile of reactivity for each score. These correlations ranged from r=−.11 to r =.33 but across tercile groups correlations were of similar magnitude and direction. No significant differences between correlations were observed between tercile groups.
Discussion
Within this sample, aggression related to poor health through higher heart rate and higher psychophysiological reactivity. Aggression itself though was related to lower heart rate and lower reactivity. These relationships suppressed the correlation between aggression and health as demonstrated by significant mediation analyses for both HR level and cardiovascular reactivity. Furthermore, individual differences in HR level moderated the Aggression/Health relationship: individuals with low HR failed to show a correlation between Aggression and Health although those with relatively high HR did. Reactivity was somewhat less strongly related to Health than HR and no clear evidence of moderation of the Aggression to Health relationship by individual differences in Reactivity were observed.
The current results related aggression to health while verifying the association between low HR and aggression in black and white men (Latvala et al., 2015; Lorber, 2004). The low, but significant correlation between aggression and HR indicates that some but not all aggressive individual have low HR. Only aggressive individuals with relatively higher heart rate show a relationship between their aggressive tendencies and poor health. The results for these individuals support prior reports (Barefoot et al., 1989; Chida & Hamer, 2008; Iribarren et al., 2005; Iribarren et al., 2000; Suarez et al., 1998; Suarez & Williams, 1989, 1990) suggesting that higher cynicism, aggression, and hostility are related to greater cardiovascular risk and overall mortality.
Individual differences in cardiovascular reactivity were related to poor health; greater reactivity was related to poorer health. Aggression, however, was significantly but not strongly related to lower cardiovascular reactivity. Again some individuals high in aggression showed low cardiovascular reactivity and hence did not show the positive association between aggression and poor health shown by others. This suppressor effect on the aggression/health relationship was not as large as that for HR, and, interestingly, HR level was uncorrelated with all measures of cardiovascular reactivity. This indicates an independence of the two suppressor effects. Notably, the current relationship of high aggression and low cardiovascular reactivity is inconsistent with a number of other reports (Chida & Steptoe, 2009). The discrepancy may be due to either: a) the characteristics of our stress tasks which differed from those in much of the literature on aggression/hostility in that they did not attempt to elicit hostility/aggression or b) sample composition in that current sample is relatively unique given the selection for anti-social behavior in a third of the sample and the high percentage of African Americans.
Interpreting the moderation by HR level of the aggression to health relationship is challenging as the empirically robust association between aggression and low HR is not completely understood. Those with primary interests in aggression and antisocial behavior have equated HR with a general physiological arousal e.g., (Scarpa, 2015). HR has been interpreted as indicative of a global physiological state, arousal, which facilitates aggressive behavior. Low HR is equated with low arousal that then is a state consistent with fearlessness. Alternatively, low arousal is interpreted as aversive leading to the individual to seek stimulation to relieve this state. Portnoy et al. (2014) review these interpretations and provide a model suggesting that impulsive sensation-seeking and not fearlessness partially mediates the relationship between low HR and aggression/non-violent delinquency, see also (Glenn & Raine, 2014). Support for either the fearlessness or sensation seeking perspective though can be questioned. The concept of low arousal if defined as a physiological state common to all indices of sympathetic activation and parasympathetic de-activation can be generally questioned (Venables, 1984; Lacey, 1967; Jennings, 1986; Berntson, Norman, Hawkley, & Cacioppo, 2008; Raine, 2002). In the current data, for example, blood pressure as a second indicator of low arousal was uncorrelated with either aggression or hostility. Furthermore, SBP reactivity was actually lower in those with higher aggression or hostility. Low HR may specifically indicate greater parasympathetic cardiac influence among those with hostile/aggressive tendencies. Prior work, however, has not been entirely consistent with this view; Raine (2002) reviews earlier findings and later work continues to show equivocal relationships. For example, Beauchaine, Hong, and Marsh (2008) find that Child Behavior Checklist rated aggression is negatively related to high frequency heart rate variability (HF-HRV), a direct index of parasympathetic control of heart, while Gordis, Feres, Olezeski, Rabkin, and Trickett (2010) find a similar relationship but only in children experiencing earlier maltreatment. In contrast, Scott and Weems (2014) found that greater HR variability was related to greater self-rated aggression, but only while participants are performing a task, see also, (Sijtsema, Roon, Groot, & Riese, 2015). We found that lower SBP and HR reactivity did tend to relate to higher aggression or hostility. Basal HR relationships are substantially more consistent across studies than either reactivity indices or the HR variability indices derived to more directly measure parasympathetic control. HR level appears as a consistent trait of the more aggressive individuals while the patterning of other autonomically controlled effectors seems dependent either on the exact context in which the measurements were made or on the specific definition of aggression/impulsivity/cynicism employed.
Some studies have examined HR levels and aggression from a developmental perspective. In a large study of Dutch adolescents parent-reported stress exposure of the adolescents was related to subsequent mental health problems, but only among those with higher HR; low HR appeared protective (Oldehinkel, Verhulst, & Ormel, 2008). This suggests a stress resiliency that may be relevant to the seeming immunity to hostility-related risk. The resiliency is lost in individuals with high HR. In our results those with high hostility and high HR did show greater SBP levels, consistent with studies relating hostility/cynicism to cardiovascular disease (Chida & Steptoe, 2009; Suarez et al., 1998; Suarez & Williams, 1989, 1990). Mediation of the HR/aggression relationship by sensation seeking was observed in another large Dutch adolescent sample, but this occurred only in boys (Sijtsema et al., 2010). Choy et al. (2015) present cross-sectional results from a large sample of 11 and 12 year old children that are modelled to suggest social adversity leading to a lowering of HR that then relates to aggression/anti-social behavior. The suggestion that lowered HR precedes the emergence of aggression/anti-social behavior is of great interest, but was not directly tested in the Choy et al. report and cannot be addressed in the current cross-sectional data. The speculation that social adversity triggers low HR might be contrasted with the position that genetic predisposition rather than early social environment may induce low HR and/or aggression, e.g., (Mick et al., 2014). We did execute an SEM model in which HR was diagrammed as leading to aggression/hostility but the fit of this model was somewhat poorer than our basic model in which aggression/hostility was diagrammed as leading to/associated with low HR. Cross-sectional data, however, cannot distinguish between these possibilities in any case. Furthermore, separating genetic predispositions for HR level (Golosheykin, Grant, Novak, Heath, & Anokhin, 2016) and aggression/hostility would seem to be difficult even with longitudinal data. The linkage of low HR to less health risk is not examined in the majority of these studies although it’s conceivable that the stress resilience suggested by the Oldehinkel et al. (2008) might be health protective.
The current findings suggest that aggression influences health differently in different individuals. The relative absence of health risk among those with low heart rate seemed independent of the relative absence of risk among those with low cardiovascular reactivity. This and the remaining pattern of results in this study and the literature question the view that a general arousal state accounts for sensation seeking or fearlessness and hence aggression. Low HR and low cardiovascular reactivity might emerge early in the life span, but we do not know yet the relative contributions of genetics and early life experience. The relationship of low HR and aggression is robust and examining its origin may be more productive than examining equivocal relationships with cardiovascular reactivity. Further understanding of both relationships now appears important, however, for promoting both health and prosocial behavior.
There are clear limitations to the current results. The data are cross-sectional and can only show concurrent associations. Participants are at an adult age at which risk can be assessed, but at which actual risk of disease incidence is low. The measures of aggression and hostility were self-reports and actual behavior or observations of others might present different information. Although the sample size was reasonably large, the detection of risk at this age is necessarily detecting relatively small effects that might be assessed more thoroughly with larger sample sizes. The sample is only male and though findings may not apply to females (Louise et al., 2012), however, the relationship between low HR and aggression is also present in females (Raine, 2002). Most importantly, this sample is likely to be particularly sensitive to correlates of aggression because of the initial selection of one third of the sample to be at risk for anti-social behavior.
In sum, the presence of low HR among aggressive and hostile individuals appears to be protective relative to health risk at early middle age. Aggression and hostility, however, overall have a deleterious influence on health, but one that is notable only among individuals with higher HR and possibly greater HR and blood pressure reactivity. The natural history of cardiovascular risk in the individual with hostility/aggression and low HR differs from the natural history of those without these characteristics. Acknowledging this difference, permits improved evaluation of risk and prevention. Further work, however, will be required to understand the source of these individual differences and their relationships to health.
Supplementary Material
Acknowledgments
This research was supported by HL111802. We acknowledge the assistance of Paul W. Scott with the statistical analysis. We also wish to thank the participants in the Pittsburgh Youth Study and the staff completing the PATHs study.
Footnotes
Review of this paper was handled by the Editor of the journal given that one of the authors (JRJ) is an Associate Editor of the journal.
References
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom W, Williams RB. The Cook-Medley Hostility scale: Item content and ability to predict survival. Psychosomatic Medicine. 1989;51(1):46–57. doi: 10.1097/00006842-198901000-00005. http://dx.doi.org/10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(7):788–796. doi: 10.1097/CHI.0b013e318172ef4b. http://dx.doi.org/10.1097/CHI.Ob013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(4):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. http://dx.doi.org/10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce W, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B, Wara D. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine. 1995;57(5):411–422. doi: 10.1097/00006842-199509000-00001. http://dx.doi.org/10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Boyce W, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. http://dx.doi.org/10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The Aggression Questionnaire. Journal of Personality and Social Psychology. 1992;63(3):452–459. doi: 10.1037//0022-3514.63.3.452. http://dx.doi.org/10.1037/0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134(6):829–885. doi: 10.1037/a0013342. http://dx.doi.org/10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. The Association of Anger and Hostility With Future Coronary Heart Disease: A Meta-Analytic Review of Prospective Evidence. Journal of the American College of Cardiology. 2009;53(11):936–946. doi: 10.1016/j.jacc.2008.11.044. http://dx.doi.org/10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Choy O, Raine A, Portnoy J, Rudo-Hutt A, Gao Y, Soyfer L. The mediating role of heart rate on the social adversity-antisocial behavior relationship: A social neurocriminology perspective. Journal of Research in Crime and Delinquency. 2015;52(3):303–341. http://dx.doi.org/10.1177/0022427814565905. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and Pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38(6):414–418. http://dx.doi.org/10.1037/h0060667. [Google Scholar]
- Costa PT, Zonderman AB, McCrae RR, Williams RB. Cynicism and paranoid alienation in the Cook and Medley HO Scale. Psychosomatic Medicine. 1986;48(3–4):283–285. doi: 10.1097/00006842-198603000-00014. http://dx.doi.org/10.1097/00006842-198603000-00014. [DOI] [PubMed] [Google Scholar]
- Fite PJ, Raine A, Stouthamer-Loeber M, Loeber R, Pardini DA. Reactive and proactive aggression in adolescent males: Examining differential outcomes 10 years later in early adulthood. Criminal Justice and Behavior. 2010;37(2):141–157. doi: 10.1177/0093854809353051. http://dx.doi.org/10.1177/0093854809353051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Phillips AC, Der G, Deary IJ, Carroll D. Heart rate reactivity is associated with future cognitive ability and cognitive change in a large community sample. International Journal of Psychophysiology. 2011;82(2):167–174. doi: 10.1016/j.ijpsycho.2011.08.004. http://dx.doi.org/10.1016/j.ijpsycho.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A. Neurocriminology: Implications for the punishment, prediction and prevention of criminal behavior. Nature Reviews Neuroscience. 2014;15(1):54–63. doi: 10.1038/nrn3640. http://dx.doi.org/10.1038/nrn3640. [DOI] [PubMed] [Google Scholar]
- Golosheykin S, Grant JD, Novak OV, Heath AC, Anokhin AP. Genetic influences on heart rate variability. International Journal of Psychophysiology. 2016 doi: 10.1016/j.ijpsycho.2016.04.008. http://dx.doi.org/10.1016/j.ijpsycho.2016.04.008. [DOI] [PMC free article] [PubMed]
- Gordis EB, Feres N, Olezeski CL, Rabkin AN, Trickett PK. Skin conductance reactivity and respiratory sinus arrhythmia among maltreated and comparison youth: relations with aggressive behavior. Journal of Pediatric Psychology. 2010;35(5):547–558. doi: 10.1093/jpepsy/jsp113. http://dx.doi.org/10.1093/jpepsy/jsp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Blood I. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Hollingshead ADB. Four factor index of social status. Yale Sociology Report. 1975;18–4 [Google Scholar]
- Iribarren C, Jacobs DR, Kiefe CI, Lewis CE, Matthews KA, Roseman JM, Hulley SB. Causes and demographic, medical, lifestyle and psychosocial predictors of premature mortality: the CARDIA study. Social Science & Medicine. 2005;60(3):471–482. doi: 10.1016/j.socscimed.2004.06.007. http://dx.doi.org/10.1016/j.socscimed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Iribarren C, Sidney S, Bild DE, Liu K, Markovitz JH, Roseman JM, Matthews K. Association of hostility with coronary artery calcification in young adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. JAMA. 2000;283(19):2546–2551. doi: 10.1001/jama.283.19.2546. http://dx.doi.org/10.1001/jama.283.19.2546. [DOI] [PubMed] [Google Scholar]
- Jennings JR. Bodily Changes during Attending. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology:Systems, Processes, and Applications. New York: Guilford; 1986. pp. 269–289. [Google Scholar]
- Jennings JR, Berg WK, Hutcheson JS, Obrist P, Porges S, Turpin G. Committee report. Publication guidelines for heart rate studies in man. Psychophysiology. 1981;18(3):226–231. doi: 10.1111/j.1469-8986.1981.tb03023.x. http://dx.doi.org/10.1111/j.1469-8986.1981.tb03023.x. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Heim AF, Kuan DC, Gianaros PJ, Muldoon MF, Manuck SB. Use of total cerebral blood flow as an imaging biomarker of known cardiovascular risks. Stroke. 2013;44(9):2480–2485. doi: 10.1161/STROKEAHA.113.001716. http://dx.doi.org/10.1161/STROKEAHA.113.001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110(15):2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. http://dx.doi.org/10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Pettersson K, Manuck SB, Olsson G. Role of sympathoadrenal medullary activation in the initiation and progression of atherosclerosis. Circulation. 1991;84(6 Suppl):VI23–32. [PubMed] [Google Scholar]
- Klabbers G, Bosma H, van den Akker M, Kempen GIJM, van Eijk JTM. Cognitive hostility predicts all-cause mortality irrespective of behavioural risk at late middle and older age. European Journal of Public Health. 2012;23(4):701–705. doi: 10.1093/eurpub/cks060. http://dx.doi.org/10.1093/eurpub/cks060. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychological Bulletin. 1984;96(3):435–464. http://dx.doi.org/10.1037/0033-2909.96.3.435. [PubMed] [Google Scholar]
- Lacey BC, Lacey JI. Studies of heart rate and other bodily processes in sensorimotor behavior. In: Obrist PA, editor. Cardiovascular psychophysiology: Current issues in response mechanisms, biofeedback and methodology. New Brunswick, NJ: AldineTransaction; US; 1974. pp. 538–564. [Google Scholar]
- Lacey JI. Somatic response patterning and stress: Some revisions of activation theory. In: Appley MH, Trumbull R, editors. Psychological Stress: Issues in Research. New York: Appleton-Century-Crofts; 1967. pp. 14–44. [Google Scholar]
- Latvala A, Kuja-Halkola R, Almqvist C, Larsson H, Lichtenstein P. A Longitudinal Study of Resting Heart Rate and Violent Criminality in More Than 700 000 Men. JAMA Psychiatry. 2015;9(1):1–8. doi: 10.1001/jamapsychiatry.2015.1165. [DOI] [PubMed] [Google Scholar]
- Loeber R, Menting B, Lynam DR, Moffitt TE, Stouthamer-Loeber M, Stallings R, Pardini D. Findings from the Pittsburgh Youth Study: Cognitive impulsivity and intelligence as predictors of the age-crime curve. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(11):1136–1149. doi: 10.1016/j.jaac.2012.08.019. http://dx.doi.org/10.1016/j.jaac.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Loeber R, Stouthamer-Loeber M, Farrington DP. The Pittsburgh Youth Study: Its design, data collection, and early key findings. In: Loeber R, Farrington DP, Stouthamer-Loeber M, White HR, editors. Violence and serious theft: Development and prediction from childhood to adulthood. New York, NY: Routledge/Taylor & Francis Group; US; 2008. pp. 25–37. [Google Scholar]
- Lorber MF. Psychophysiology of Aggression, Psychopathy, and Conduct Problems: A Meta-Analysis. Psychological Bulletin. 2004;130(4):531–552. doi: 10.1037/0033-2909.130.4.531. http://dx.doi.org/10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Louise S, Warrington NM, McCaskie PA, Oddy WH, Zubrick SR, Hands B, Beilin LJ. Associations between aggressive behaviour scores and cardiovascular risk factors in childhood. Pediatric Obesity. 2012;7(4):319–328. doi: 10.1111/j.2047-6310.2012.00047.x. http://dx.doi.org/10.1111/j.2047-6310.2012.00047.x. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Adams MR, Clarkson TB. Behaviorally elicited heart rate reactivity and atherosclerosis in female cynomolgus monkeys (Macaca fascicularis) Psychosomatic Medicine. 1989;51(3):306–318. doi: 10.1097/00006842-198905000-00005. http://dx.doi.org/10.1097/00006842-198905000-00005. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Clarkson TB. Behaviorally induced heart rate reactivity and atherosclerosis in cynomolgus monkeys. Psychosomatic Medicine. 1983;45(2):95–108. doi: 10.1097/00006842-198305000-00002. http://dx.doi.org/10.1097/00006842-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110(1):74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. http://dx.doi.org/10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Woodall KL, Stoney CM. Changes in and stability of cardiovascular responses to behavioral stress: Results from a four-year longitudinal study of children. Child Development. 1990;61(4):1134–1144. http://dx.doi.org/10.2307/1130881. [PubMed] [Google Scholar]
- Matthews KA, Zhu S, Tucker DC, Whooley MA. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2006;47(3):391–395. doi: 10.1161/01.HYP.0000200713.44895.38. http://dx.doi.org/10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- Menown IB, Davies S, Gupta S, Kalra PR, Lang CC, Morley C, Padmanabhan S. Resting heart rate and outcomes in patients with cardiovascular disease: where do we currently stand? Cardiovascular Therapeutics. 2013;31(4):215–223. doi: 10.1111/j.1755-5922.2012.00321.x. http://dx.doi.org/10.1111/j.1755-5922.2012.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkin SS, Karlamangla A, Elashoff D, Grogan T, Seeman T. Change in cardiometabolic score and incidence of cardiovascular disease: the multi-ethnic study of atherosclerosis. Annals of Epidemiology. 2015;25(12):912–917.e911. doi: 10.1016/j.annepidem.2015.09.006. http://dx.doi.org/10.1016/j.annepidem.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel AJ, Verhulst FC, Ormel J. Low heart rate: a marker of stress resilience. The TRAILS study. Biological Psychiatry. 2008;63(12):1141–1146. doi: 10.1016/j.biopsych.2007.12.006. http://dx.doi.org/10.1016/j.biopsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart Rate Level and Antisocial Behavior in Children and Adolescents: A Meta-Analysis. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(2):154–162. doi: 10.1097/00004583-200402000-00010. http://dx.doi.org/10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RSJ, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The Association Of Changes In Physical-Activity Level And Other Lifestyle Characteristics With Mortality Among Men. New England Journal of Medicine. 1993;328(8):538–545. doi: 10.1056/NEJM199302253280804. http://dx.doi.org/10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- Phillips AC. Blunted cardiovascular reactivity relates to depression, obesity, and self-reported health. Biological Psychology. 2011;86(2):106–113. doi: 10.1016/j.biopsycho.2010.03.016. http://dx.doi.org/10.1016/j.biopsycho.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, Hughes BM. The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International Journal of Psychophysiology. 2013;90(1):1–7. doi: 10.1016/j.ijpsycho.2013.02.002. http://dx.doi.org/10.1016/j.ijpsycho.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Hunt K, Der G, Carroll D. Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: Evidence from a large community study. Psychophysiology. 2011;48(1):142–148. doi: 10.1111/j.1469-8986.2010.01045.x. http://dx.doi.org/10.1111/j.1469-8986.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- Portnoy J, Farrington DP. Resting heart rate and antisocial behavior: An updated systematic review and meta-analysis. Aggression and Violent Behavior. 2015;22:33–45. http://dx.doi.org/10.1016/j.avb.2015.02.004. [Google Scholar]
- Portnoy J, Raine A, Chen FR, Pardini D, Loeber R, Jennings J. Heart rate and antisocial behavior: The mediating role of impulsive sensation seeking. Criminology: An Interdisciplinary Journal. 2014;52(2):292–311. http://dx.doi.org/10.1111/1745-9125.12038. [Google Scholar]
- Raine A. Annotation: The role of prefrontal deficits, low autonomic arousal and early health factors in the development of antisocial and aggressive behavior in children. Journal of Child Psychology and Psychiatry. 2002;43(4):417–434. doi: 10.1111/1469-7610.00034. http://dx.doi.org/10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- Raine A, Fung ALC, Portnoy J, Choy O, Spring VL. Low heart rate as a risk factor for child and adolescent proactive aggressive and impulsive psychopathic behavior. Aggressive Behavior. 2014;40(4):290–299. doi: 10.1002/ab.21523. http://dx.doi.org/10.1002/ab.21523. [DOI] [PubMed] [Google Scholar]
- Scarpa A. Physiological arousal and its dysreglation in child maladjustment. Current Directions in Psychological Science. 2015;24(5):345–351. http://dx.doi.org/10.1177/0963721415588920. [Google Scholar]
- Scott BG, Weems CF. Resting vagal tone and vagal response to stress: Associations with anxiety, aggression, and perceived anxiety control among youths. Psychophysiology. 2014;51(8):718–727. doi: 10.1111/psyp.12218. http://dx.doi.org/10.1111/psyp.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler IC, Peterson BL, Barefoot JC, Williams RB. Hostility during late adolescence predicts coronary risk factors at mid4life. American Journal of Epidemiology. 1992;136(2):146–154. doi: 10.1093/oxfordjournals.aje.a116481. [DOI] [PubMed] [Google Scholar]
- Sijtsema JJ, Roon VA, Groot FP, Riese H. Early Life Adversities and Adolescent Antisocial Behavior: The Role of Cardiac Autonomic Nervous System Reactivity in the TRAILS Study. Biological Psychology. 2015;110:24–33. doi: 10.1016/j.biopsycho.2015.06.012. http://dx.doi.org/10.1016/j.biopsycho.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Sijtsema JJ, Veenstra R, Lindenberg S, van Roon AM, Vernulst FC, Ormel J, Riese H. Mediation of sensation seeking and behavioral inhibition on the relationship between heart rate and antisocial behavior: The TRAILS study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(5):493–502. doi: 10.1097/00004583-201005000-00010. http://dx.doi.org/10.1097/00004583-201005000-00010. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Jr, Zimmermann EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: The role of interpersonal challenge. Psychosomatic Medicine. 1998;60(1):78–88. doi: 10.1097/00006842-199801000-00017. http://dx.doi.org/10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Williams RB. Situational determinants of cardiovascular and emotional reactivity in high and low hostile men. Psychosomatic Medicine. 1989;51(4):404–418. doi: 10.1097/00006842-198907000-00004. http://dx.doi.org/10.1097/00006842-198907000-00004. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Williams RB. The relationships between dimensions of hostility and cardiovascular reactivity as a function of task characteristics. Psychosomatic Medicine. 1990;52(5):558–570. doi: 10.1097/00006842-199009000-00008. http://dx.doi.org/10.1097/00006842-199009000-00008. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine. 2003;65(1):46–62. doi: 10.1097/00006842-200301000-00007. http://dx.doi.org/10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Venables PH. Arousal: An examination of its status as a concept. In: Coles MGH, Jennings JR, Stern JA, editors. Psychophysiological Perspectives: Festschrift for Beatrice and John Lacey. New York: Van Nostrand Reinhold; 1984. pp. 134–142. [Google Scholar]
- Wong JM, Sin NL, Whooley MA. A comparison of cook-medley hostility subscales and mortality in patients with coronary heart disease: data from the heart and soul study. Psychosomatic Medicine. 2014;76(4):311–317. doi: 10.1097/PSY.0000000000000059. http://dx.doi.org/10.1097/PSY.0000000000000059. [DOI] [PubMed] [Google Scholar]
- Zahn TP. Psychophysiological approaches to psychopathology. In: Coles MGH, Donchin M, Porges SW, editors. Psychophysiology: Systems, Processes, and Applications. New York: Guilford; 1986. pp. 508–610. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


