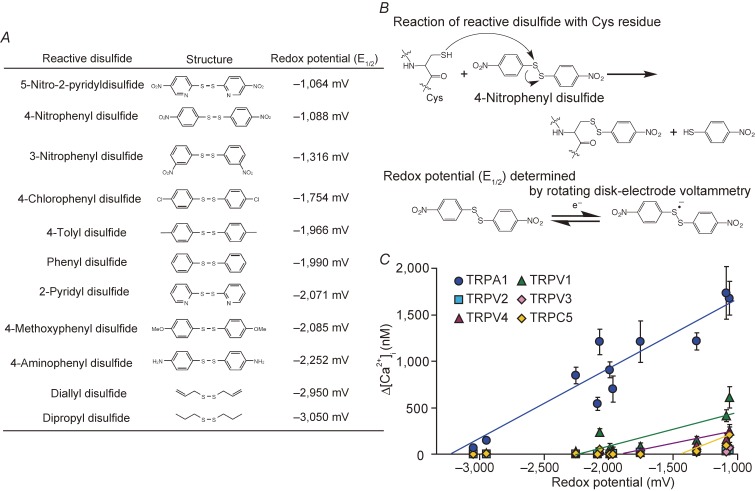
Figure 1.
Quantification of oxidation sensitivity of TRP channels. A) Chemical structures of reactive disulphides and the redox potentials (E1/2) determined by rotating disk-electrode voltammetry. E1/2 is an empirical value that is defined as the midpoint of the rise of current in voltammogram and, as such, it differs from the standard reduction potential (E0) of the compound. E1/2 values of 5 mM reactive disulphides dissolved in dehydrated DMSO were measured in 0.1 M Bu4NBF4/DMSO using a glassy carbon working electrode, a platinum wire counter electrode and an Ag/Ag+ reference electrode at room temperature. Modified from Takahashi et al.90) B) The chemical reaction of a reactive disulphide compound with a Cys sulfhydryl (upper) and the single electron redox reaction, for which we determined E1/2 values by rotating disk-electrode voltammetry (lower). C) Oxidation sensitivity of TRP channels. Plots of maximum [Ca2+]i rises (Δ[Ca2+]i) induced by 10 µM reactive disulphides in HEK293 cells expressing redox-sensitive TRP channels against redox potentials of respective substances described in A). Modified from Takahashi et al.90)