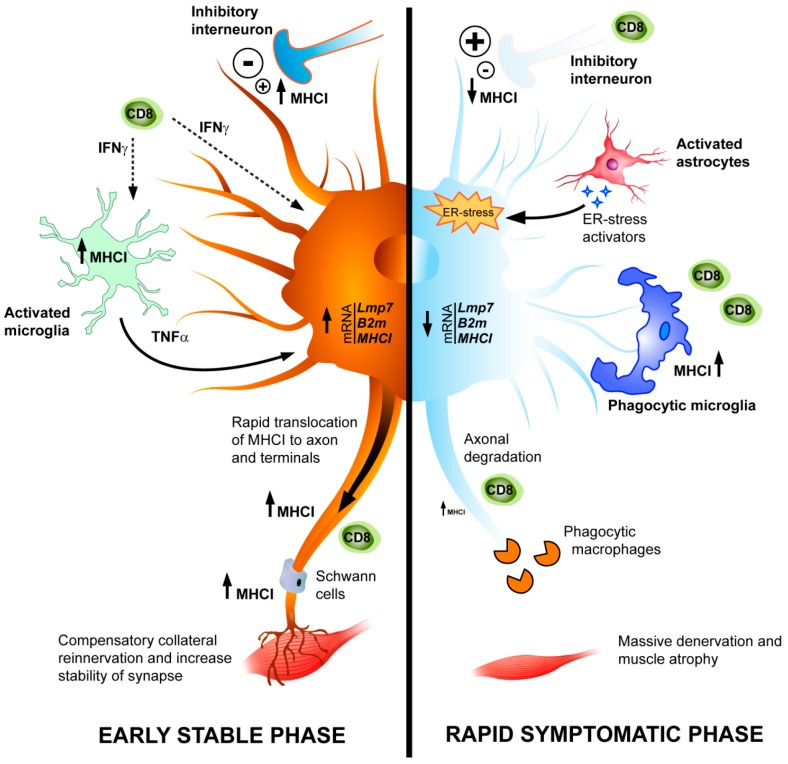
Figure 2.
Schematic representation of dysregulation of MHCI in MNs during ALS pathology. Left side: in SOD1G93A mice, at the early phase of the disease, a marked upregulation of mRNAs for MHCI and associated components, immunoproteasome (LMP7) and β2 microglobulin (β2m), occurs in MNs in response to accumulation of misfolded proteins (i.e., mutant SOD1) and presumably to the release of pro-inflammatory cytokines from activated microglia and astrocytes. Once transduced, MHCI is rapidly translocated to motor axons and terminals leaving the soma almost deprived of MHCI protein. This is associated with recruitment of T cells in sciatic nerves and axon terminals. Since the retraction of damaged motor axons at the neuromuscular junctions is the earliest event in ALS, we hypothesize that the upregulation of MHCI in the periphery may activate cytotoxic T cells to create a growth-permissive milieu, which promotes the pruning of damaged motor axons and the compensatory collateral reinnervation of muscles. In addition, MHCI maintains the proper activity of Schwann cells (SCs) in the motor axons and terminal Schwann cells (tSCs) at the neuromuscular junctions providing stability and synapse homeostasis. Right side: at the symptomatic phase, the highly reactive astrocytes around MNs contribute significantly to the reduction of MHCI through the activation of MHCI inhibitory receptor. A sustained immunoreactivity for MHCI persists in microglia with a phagocytic phenotype. The levels of MHCI, which is nearly absent in the MN perikarya, decrease also in motor axons and terminals. The skeletal muscles are massively denervated at this stage resulting in loss of function, atrophy, and motor paralysis. The activated microglia in the spinal cord overexpressing MHCI may increase the recruitment of CD8+ T cells contributing to the removal of damaged MNs in concert with macrophages at the final stage. ↑ upregulation; ↓ downregulation; Dashed line arrow—secretion of soluble factor; Long continuous arrow: effects of activated glia.