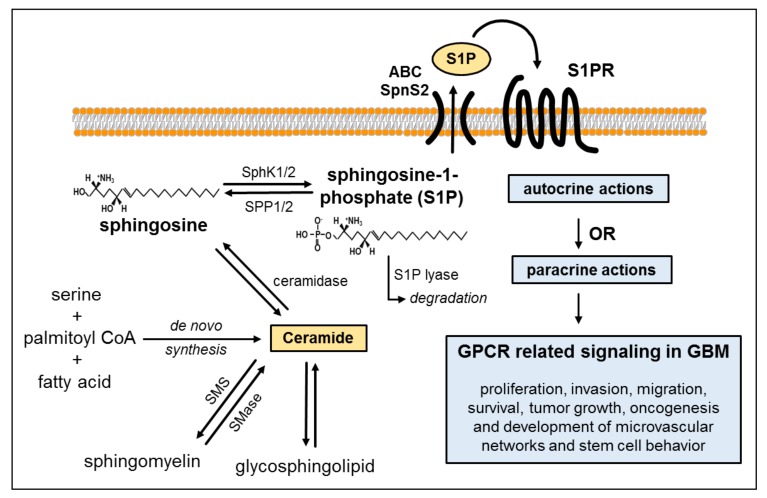
Figure 1.
Biosynthesis of ceramide and sphingosine-1-phosphate (S1P) production. Ceramide de novo synthesis typically originates from the condensation of serine, palmitoyl-CoA and fatty acids—a multistep enzyme-catalyzed process. Ceramide can be transformed reversibly (indicated by the two-way arrows) into sphingomyelin by sphingomyelinase or to glycosphingolipids. It is further metabolized to sphingosine by ceramidase. Sphingosine can then be phosphorylated into S1P by the sphingosine kinase isoforms 1 and 2 (SphK1/2). This phosphorylation can be reverted by the S1P phosphatases 1 and 2 (SPP1/2), or irreversible degradation by S1P lyase can occur. S1P produced intracellularly is exported out of the cell via ATP-binding cassette transporters (ABC) transporters or spinster homolog 2 (Spns2), dependent on the cell type. Subsequently, it can act either in an autocrine or paracrine manner by binding to one of its receptors (S1PR1–5) to regulate multifaceted cellular functions via G-protein-mediated signaling. S1P promotes key processes of glioblastoma multiforme (GBM) pathogenesis which involve cell proliferation, invasion, migration, survival, tumor growth, oncogenesis and development of microvascular networks. Additional abbreviations used in the figure are defined as follows: SMase, sphingomyelinase; SMS, sphingomyelin synthase; S1PRs, S1P receptor(s).