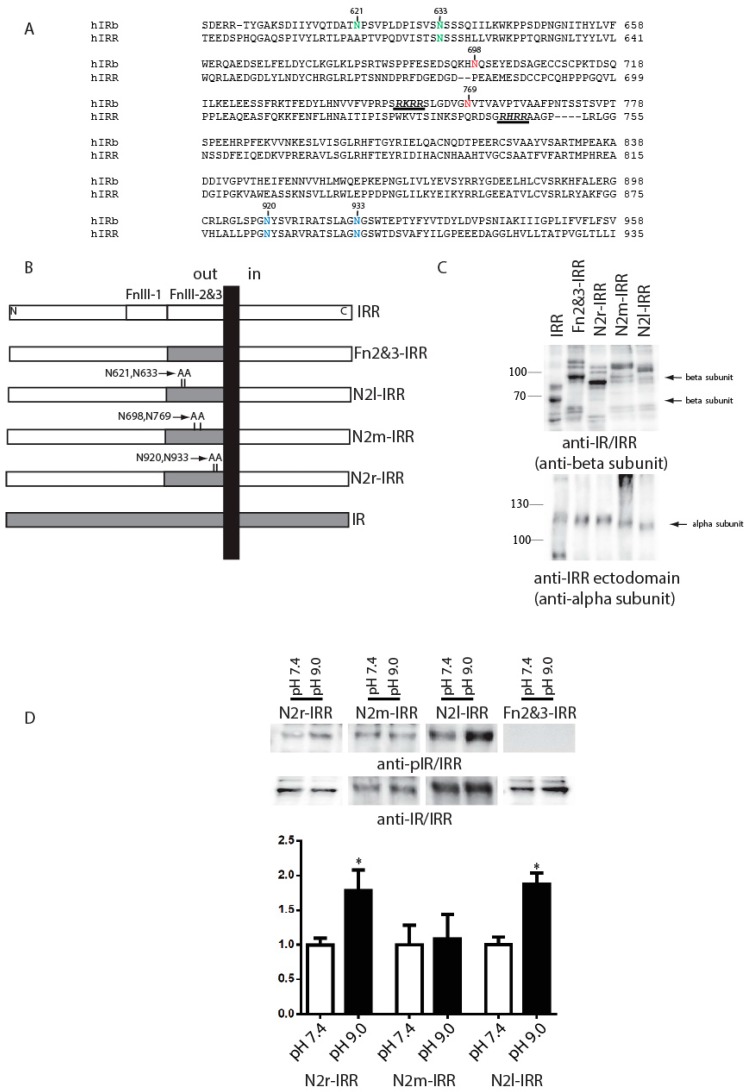
Figure 4.
The role of glycosylation within fibronectin repeats domains. (A) Alignment of human IR isoform b and human IRR sequences with indication of mutated resides. N2l (left), N2m (middle) and N2r (right) mutations indicated by green, red, or blue colors, correspondently. Sites of proteolysis also indicated by underlying; (B) Domain models of the HA-tagged Fn2&3-IRR, N2l-IRR, N2m-IRR, and N2r-IRR alanine mutants or chimeras; (C) Western blotting of indicated constructs with anti-IR/IRR antibody (against C-end of IRR) and with anti-IRR ectodomain antibody (against alpha-subunit of IRR); (D) Quantitative analysis of the activation of indicated constructs by alkali. For each constructs basal phosphorylation at pH 7.4 indicated as 100%. Transfected cells were incubated with two set of Tris-buffered physiological saline solutions with pH from 7.4 or 9.0. Lysates of transfected cells were directly analyzed by Western blotting with anti-pIR/IRR antibodies and after stripping with anti-IR/IRR antibodies. For the quantitative analysis of Western blots we used Fusion Solo system (Vilber Lourmat, France). Asterisks indicate p < 0.05 in comparison with the basal phosphorylation at pH 7.4 of same constructs. Values are means ± SE (n ≥ 4).