Graphical Abstract
Five homochiral open-framework materials based on manganese and magnesium camphorates exhibit homochiral connectivity from one to three dimensions, highlighting the versatility of the synthetic system reported here. Two compounds contain column of homochiral chains lining up the honeycomb channels of 3-D metal-oxygen frameworks, an unusual feature previously unobserved in inorganic-organic hybrids.

Hybrid organic–inorganic coordination assembly, which may incorporate functionality from both inorganic and organic components, is currently an active research area.1–3 However, despite impressive progress in the past decade, there remain a number of challenges, two of which are highlighted here. One is the control of the dimensionality in inorganic connectivity. 2–5 Inorganic components in the majority of coordination polymers are isolated metal cations or clusters and only a very limited number of 3-D inorganic connectivity (within organic-inorganic hybrids) are known.3, 4 The 3-D inorganic connectivity is desirable for properties resulting from cooperative phenomenon such as magnetism 3c and conductivity.2d
The second challenge relates to homochiral open-framework materials that have potential enantioselective applications. 6–8 While the crystallization of chiral crystals from achiral precursors is not uncommon, the bulk sample tends to be a racemate, except in some rare cases when a particular chiral form is preferentially formed. 7 For enantioselective applications, it is desirable to develop new synthetic procedures to produce enantiopure open-framework materials.
In known 3-D homochiral crystalline inorganic-organic hybrids, enantiopure ligands generally serve as structural building units to crosslink inorganic units into covalent frameworks, and chiral ligands are essential for the 3-D framework connectivity. The framework dimensionality in terms of the covalent connectivity would generally be lowered (to 2, 1, or 0-dimension) without crosslinking chiral ligands.
Here we present two isostructural homochiral materials (1 and 2) in which homochiral features serve as decoration on 3-D metal-oxygen frameworks. The most interesting feature is that the 1-D homochiral chains can be considered not as a part of the 3-D framework, but as decorative ligands to strengthen the existing 3-D metal-oxygen framework. Compounds 1 and 2 are also among rare inorganic-organic hybrids that contains 3-D inorganic connectivity (here, 3-D Mn-O or Mg-O network). It is worth noting that the 3-D inorganic connectivity among homochiral inorganic-organic hybrids is rare. Some metal carboxylates with 3-D M-O-M connectivity are known, but they are not homochiral.[2c, 3a, 5a]
Compounds 1 and 2 are among a total of five homochiral framework materials prepared in this work. These five compounds possess homochiral connectivity (i.e. the connectivity between enantiopure ligands and metal centers) in 1-, 2- and 3-dimensions. Such diverse homochiral features highlight the versatility of the synthetic system reported here. While 1, 3, 4, and 5 are based on the magnetic Mn2+ cations, we also prepared 2 to demonstrate that the synthetic chemistry reported here can be extended to other metal species such as Mg2+.
Homochiral materials reported here are based on D-camphoric acid (D-H2Cam) (Table 1). Figure 1a shows an unusual [Mn3(HCOO)4]n2n− 3-D framework in 1 with 3-D Mn-O-Mn connectivity and open honeycomb channels. The organic chains based on enantipure D-Cam ligands are attached to the wall of the channels, generating an unprecedented framework with 3-D inorganic M-O connectivity and decorative 1-D chiral chains (Figures 1a–e). Each Mn2+ site in 1 has distorted octahedral geometry. There are three independent Mn2+ ions. Mn1 and Mn2 are connected by HCOO− ligands to form a 32 helix along the c axis. Each 32 helix is connected to three adjacent helices by Mn3. Such connectivity results in the formation of the [Mn3(HCOO)4]n 3-D framework with open channels along the c axis (Figure 1a). The diameter of the cylindrical channel is about 14 Å.
Table 1.
A Summary of Crystal Data and Refinement Results.
| Formula | S. G. | a (Å) | b (Å) | c (Å) | β (°) | R(F) | Flack parameter | CnAm type | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | [Mn3(HCOO)4(D-Cam)]n | P32 | 15.128(1) | 15.128(1) | 7.716(1) | 90 | 0.0662 | 0.09(5) | C1A3 |
| 2 | [Mg3(HCOO)4(D-Cam)]n | P32 | 14.872(1) | 14.872(1) | 7.333(1) | 90 | 0.0751 | 0.02(7) | C1A3 |
| 3 | [Mn2(D-Cam)2(DMA)2]n | P21 | 9.670(1) | 13.269(1) | 13.274(1) | 108.63(1) | 0.0847 | 0.2(3) | C2A0 |
| 4 | [Mn3(HCOO)2(D-Cam)2(DMF)2]n | P21 | 8.872(1) | 13.708(1) | 14.441(1) | 97.75(1) | 0.0646 | 0.01 (1) | C2A1 |
| 5 | [Mn2(D-Cam)2]n | P21 | 6.868(1) | 12.566(2) | 12.782(2) | 103.46(1) | 0.0389 | −0.04(2) | C3A0 |
D-H2Cam = D-camphoric acid; DMF = N,N′-dimethyl formamide; DMA = N,N′-dimethyl acetamide; S.G. = Space Group; CCDC-655527 - 655531 (1–5)
Figure 1.
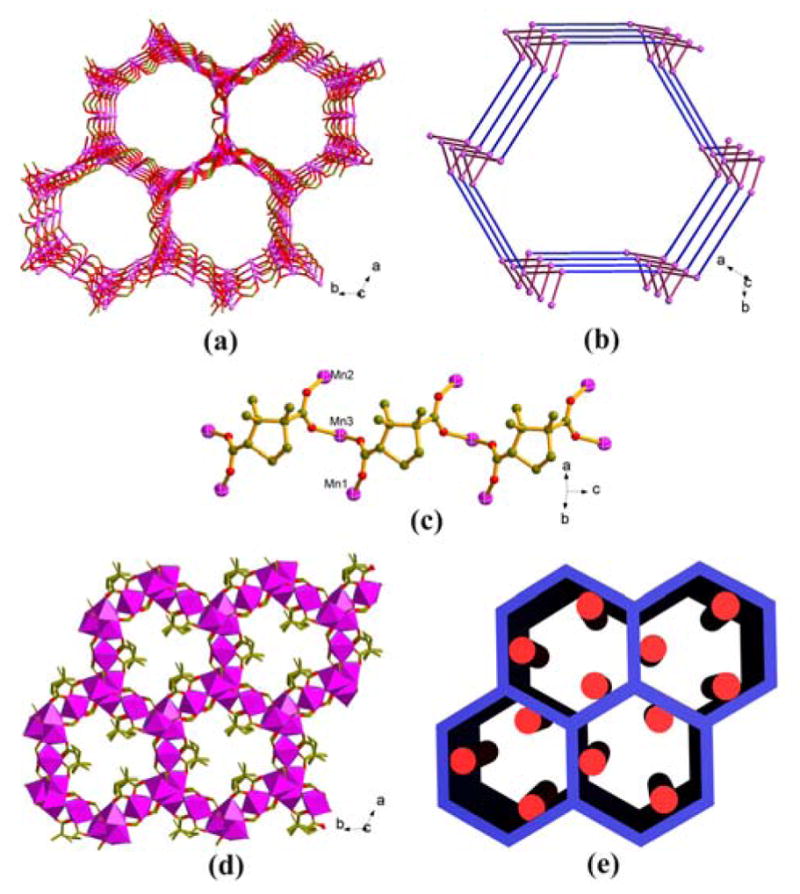
(a) The [Mn3(HCOO)4]n2n+ 3D framework with 3D Mn-O-Mn connectivity and open channels along c axis; (b) topological representation of the eta net in 1; (c) the D-Cam ligands link trinuclear Mn(II) centers into 1D helical chain in 1; (d) the 3D I3O1 (or C1A3) framework of 1, showing the 3D Mn-O-Mn connectivity (polyhedron) and the attached homochiral chains; (e) schematic representation of the honeycomb-like 3D frameworks with attached 1D homochiral chains.
The simplification of the 3-D network in 1 by connecting all the Mn2+ sites gives a distorted eta net (or (8,3)-a net) 9 where the Mn1 centers behave as 3-connected nodes (Figure 1b). In this net, the linkage between the 32 helices with the same handedness gives rise to a 3-D network. All four independent HCOO− ligands use μ2-O atoms to bridge Mn2+ ions, resulting in an 3-D Mn-O-Mn framework. Thermal analysis results show compound 1 has relatively high stability with no weight loss under 400 °C. Magnetic susceptibility measurements reveal dominant antiferromagnetic behavior and the magnetic data above 25 K can be fitted to the Curie–Weiss law with C = 11.18 cm3 K mol−1 and θ = −61.13 K.
The large space of each hexagonal channel accommodates three columns of the D-Cam ligands with all chiral C centers of the D-Cam ligands directed towards the centre of the channel. Such exposed chirality centers are particularly desirable for chiral recognition, but unfortunately, no additional solvent accessible space is present within the honeycomb channels because channels are already filled with columns of homochiral ligands.
By employing different solvents, three other homochiral compounds 3–5 were obtained. Unlike compound 1 with 1-D homochiral connectivity (attached to 3-D M-O-M framework), compounds 3–4 exhibit 2-D homochiral connectivity while compound 5 has 3-D homochiral connectivity. The n-D homochiral connectivity means that metal cations or metal clusters are joined together by enantiopure ligands in n-dimensions. The dimensionality of homochiral connectivity may be equal or lower than the overall framework dimensionality because of the additional crosslinking by achiral ligands.
It can be useful to indicate the dimensionality of metal/chiral-ligand connectivity and the dimensionality of metal/achiral-ligand connectivity using the CnAm scheme first proposed here, where C and A represent chiral and achiral connectivity, respectively and n and m represent dimensionality of chiral and achiral connectivity. The CnAm scheme is similar to the previously reported InOm scheme that shows inorganic and organic connectivity in inorganic-organic hybrids. 2a
Compound 3 has a homochiral 2-D layered structure with dinuclear Mn units bridged by the D-Cam ligands and belongs to the C2A0 type structure (Figure 2a). Each Mn(II) site in 3 has square pyramidal geometry and the Mn dimers are bridged by four bidentate carboxylate groups from four D-Cam ligands into a paddle-wheel. The DMA molecule affords one oxygen atom to complete the five-coordinate geometry of the Mn(II) center.
Figure 2.
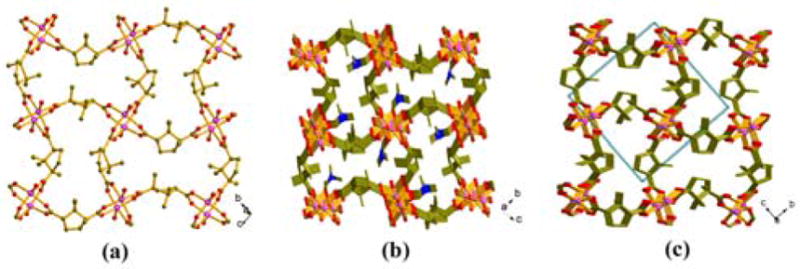
(a) The 2D homochiral layer in 3; (b) the 3D C2A1 framework of 4; (c) the 3D C3A0 framework of 5.
Compound 4 consists of homochiral layers linked by 1-D achiral chains and therefore has a C2A1 3-D framework (Figure 2b). The D-Cam ligands act as μ4-linkers and connect the trinuclear Mn units to form a homochiral (4,4) layer parallel to the bc plane. Both DMF and HCOO− ligands afford μ2-O atoms to connect Mn(II) centers to form a 1-D Mn-O-Mn chain along the a axis. The homochiral layer and achiral Mn-O-Mn chain share the common trinuclear Mn units resulting in a 3-D C2A1 homochiral framework (n + m =3).
Compound 5 is formed between Mn2+ ions and D-Cam ligands and has the 3-D homochiral connectivity (C3A0) (Figure 2c). It consists of Mn2+ chains bridged by homochiral D-Cam ligands. There are two independent Mn2+ centers and both of them are coordinated by five carboxylate oxygen atoms from four D-Cam ligands in a distorted square pyramidal geometry. Mn1 and Mn2 atoms are bridged by two carboxylate groups from two independent D-Cam ligands to form a chain with corner-sharing [MnO5] square pyramids. Each carboxylate-bridged Mn chain is further linked to four neighboring chains by D-Cam ligands, to generate a 3-D homochiral framework. Such framework can be described as the PtS net by considering Mn2+ as tetrahedral nodes and D-Cam as planar 4-connected nodes.
In conclusion, we have synthesized five framework solids that exhibit homochiral connectivity from 1-, 2- to 3-dimensions. The overall framework connectivity of these materials ranges from 2 to 3-dimensions by also considering achiral connectivity. Compounds 1 and 2 are unusual because of the presence of 3-D inorganic metal-oxygen frameworks coupled with the decoration of their honeycomb channels by columns of homochiral chains.
Supplementary Material
Acknowledgments
We thank the support of this work by the NIH (2 S06 GM063119-05), NSF-MRI, NIH-RISE, and the SCAC award (Summer 2007) of CSULB.
Footnotes
Supporting Information Available: Detailed synthesis conditions, additional structural diagrams, thermal analysis data, experimental and simulated X-ray powder diffraction patterns, magnetic data, and CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Nature. 2003;423:705. doi: 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]; (b) Kitagawa S, Kitaura R, Noro S. Angew Chem Int Ed. 2004;43:2334. doi: 10.1002/anie.200300610. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cheetham AK, Rao CNR, Feller RK. Chem Commun. 2006:4780. doi: 10.1039/b610264f. [DOI] [PubMed] [Google Scholar]; (b) Guillou N, Gao QM, Forster PM, Chang JS, Park SE, Férey G, Cheetham AK. Angew Chem Int Ed. 2001;40:2831. doi: 10.1002/1521-3773(20010803)40:15<2831::AID-ANIE2831>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]; (c) Forster PM, Cheetham AK. Angew Chem Int Ed. 2002;41:457. doi: 10.1002/1521-3773(20020201)41:3<457::aid-anie457>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]; (d) Ferey G, Millange F, Morcrette M, Serre C, Doublet M-L, Greneche J-M, Tarascon J-M. Angew Chem Int Ed. 2007;46:3259. doi: 10.1002/anie.200605163. [DOI] [PubMed] [Google Scholar]
- 3.(a) Livage C, Forster PM, Guillou N, Tafoya MM, Cheetham AK, Férey G. Angew Chem Int Ed. 2007;46:5877. doi: 10.1002/anie.200700247. [DOI] [PubMed] [Google Scholar]; (b) Serre C, Millange F, Surble S, Greneche JM, Férey G. Chem Mater. 2004;16:2706. [Google Scholar]; (c) Guillou N, Livage C, Drillon M, Férey G. Angew Chem Int Ed. 2003;42:5314. doi: 10.1002/anie.200352520. [DOI] [PubMed] [Google Scholar]; (d) Livage C, Egger C, Férey G. Chem Mater. 2001;13:410. [Google Scholar]
- 4.Gutschke SOH, Price DJ, Powell AK, Wood PT. Angew Chem Int Ed. 2001;40:1920. [PubMed] [Google Scholar]
- 5.(a) Wang Z, Zhang B, Fujiwara H, Kobayashi H, Kurmoo M. Chem Commun. 2004:416. doi: 10.1039/b314221c. [DOI] [PubMed] [Google Scholar]; (b) Dybtsev DN, Chun H, Yoon SH, Kim D, Kim K. J Am Chem Soc. 2004;126:32. doi: 10.1021/ja038678c. [DOI] [PubMed] [Google Scholar]
- 6.(a) Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K. Nature. 2000;404:982. doi: 10.1038/35010088. [DOI] [PubMed] [Google Scholar]; (b) Kesanli B, Lin W. Coord Chem Rev. 2003;246:305. [Google Scholar]; (c) Anokhina EV, Go YB, Lee Y, Vogt T, Jacobson AJ. J Am Chem Soc. 2006;128:9957. doi: 10.1021/ja062743b. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lin Z, Slawin AMZ, Morris RE. J Am Chem Soc. 2007;129:4880. doi: 10.1021/ja070671y. [DOI] [PubMed] [Google Scholar]; (b) Ezuhara T, Endo K, Aoyama Y. J Am Chem Soc. 1999;121:3279. [Google Scholar]
- 8.(a) Vaidhyanathan R, Bradshaw D, Rebilly JN, Barrio JP, Gould JA, Berry NG, Rosseinsky MJ. Angew Chem Int Ed. 2006;45:6495. doi: 10.1002/anie.200602242. [DOI] [PubMed] [Google Scholar]; (b) Dybtsev DN, Yutkin MP, Peresypkina EV, Virovets AV, Serre C, Férey G, Fedin VP. Inorg Chem. 2007;46:6843. doi: 10.1021/ic7009226. [DOI] [PubMed] [Google Scholar]; (c) Zhang J, Bu X. Angew Chem Int Ed. 2007;46:6115. doi: 10.1002/anie.200701374. [DOI] [PubMed] [Google Scholar]
- 9.Rosi NL, Kim J, Eddaoudi M, Chen B, O’Keeffe M, Yaghi OM. J Am Chem Soc. 2005;127:1504. doi: 10.1021/ja045123o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


