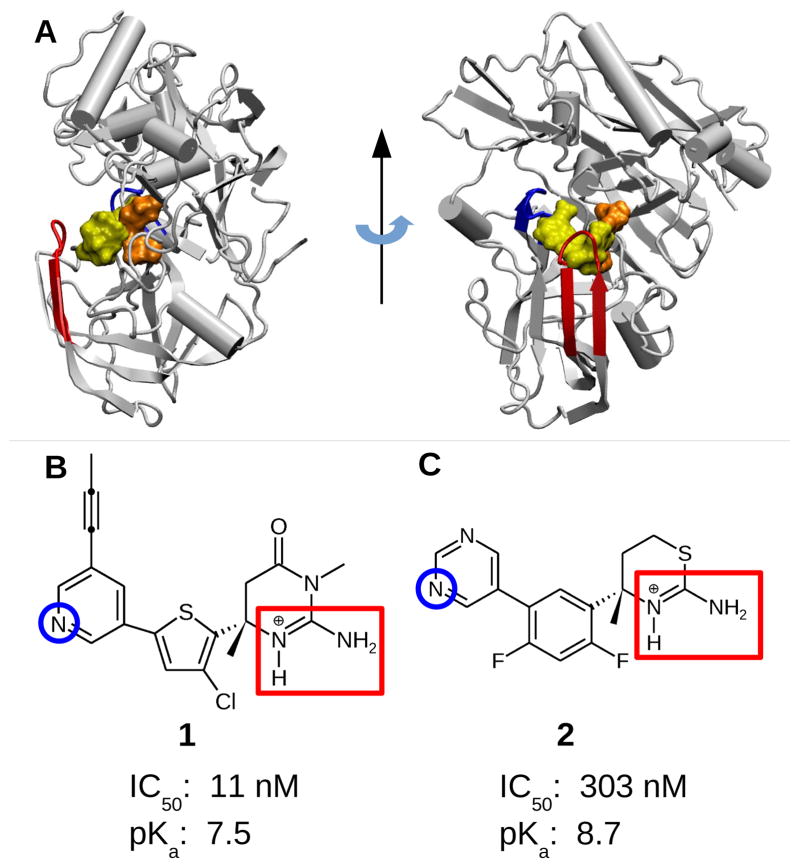
Figure 1.
Structures of BACE1 and the two small-molecule inhibitors. A. BACE1 in complex with inhibitor 1 (PDB ID 4FRS 7). The inhibitor, catalytic dyad, flap, and 10s loop are shown in yellow, orange, red, and blue, respectively. B. Iminopyrimidinone-based inhibitor 1 discovered by Merck (MK8698). 7 C. Aminothiazine-based inhibitor 2 discovered by Eli Lilly (LY2811376). 8 The two endo- and exocyclic amines on the ABM are highlighted by a red rectangle. The nitrogen on the pyridine/pyrimidine ring (titratable in the simulations) is indicated by a blue circle. Experimental cell IC50 values for 1 and 2 are taken from ref 7 and ref 8, respectively. Experimental pKa’s refer to those of the endocyclic amine group of the ABM: 1 is taken from ref 7, while 2 was obtained by capillary electrophoresis experiments (see Methods and Protocols).