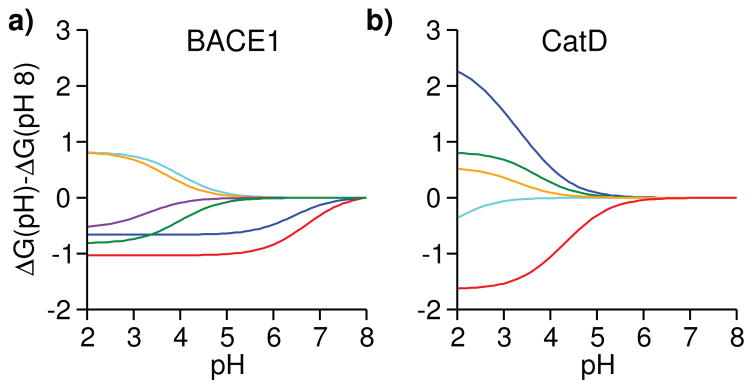
Figure 4. Residue-specific contributions to the pH-dependent binding free energies of BACE1 and CatD.
ΔG at pH 8 is used as a reference. a) Contributions from His45 and His145 to the BACE1 binding free energy are shown in red and blue, respectively. Contributions from Asp130, Asp223, Asp363 and the inhibitor titratable site are shown in cyan, orange, purple and green, respectively. The block standard error for His45 pKa shift is 0.18, which corresponds to a binding free energy error of 0.24 kcal/mol. (b) Contributions from the inhibitor titratable site and catalytic Asp33 to the CatD binding free energy are shown in red and blue, respectively. Contributions from Asp75, Asp152 and Asp231 are shown in green, cyan and orange, respectively.