Abstract
The purpose of this article was to perform a systematic review of the recent literature on urethral tissue engineering. A total of 31 articles describing the use of tissue engineering for urethra reconstruction were included. The obtained results were discussed in three groups: cells, scaffolds, and clinical results of urethral reconstructions using these components. Stem cells of different origin were used in many experimental studies, but only autologous urothelial cells, fibroblasts, and keratinocytes were applied in clinical trials. Natural and synthetic scaffolds were studied in the context of urethral tissue engineering. The main advantage of synthetic ones is the fact that they can be obtained in unlimited amount and modified by different techniques, but scaffolds of natural origin normally contain chemical groups and bioactive proteins which increase the cell attachment and may promote the cell proliferation and differentiation. The most promising are smart scaffolds delivering different bioactive molecules or those that can be tubularized. In two clinical trials, only onlay-fashioned transplants were used for urethral reconstruction. However, the very promising results were obtained from animal studies where tubularized scaffolds, both non-seeded and cell-seeded, were applied.
Impact statement
The main goal of this article was to perform a systematic review of the recent literature on urethral tissue engineering. It summarizes the most recent information about cells, seeded or non-seeded scaffolds and clinical application with respect to regeneration of urethra.
Keywords: Tissue engineering, stem cells, scaffolds, autologous grafts, urethral reconstruction, regenerative medicine
Introduction
The urethra is the part of the lower urinary tract, and in males, it is the part of the genital tract as well. It is responsible for the transport of urine from the urinary bladder to outside of the body. In males, as the part of the genital tract, it is also involved in the ejaculation of semen. Urethra in females is relatively short, but in males it reaches a length of 18–20 cm.1 Many pathological processes during all developmental stages may affect urethra and thus negatively affect the quality of life of the patients at different ages and genders. In many cases, the surgical treatment is the only option to resolve this problem.2
The most common conditions that require the operative interventions of urethra are damages caused by catheterization, pelvic trauma, infection, systemic disease – lichen sclerosus, etc., leading to the formation of strictures. Strictures most often occur as a result of scarring, which replaces the vascular tissue of the corpus spongiosum, leading to ischemic spongiofibrosis of the urethra. Replacement of damaged urethra by scar tissue leads to a reduction of its lumen, with the gradual formation of lower urinary tract obstruction. Chronic obstruction of the lower urinary tract may result in urinary retention, loss of bladder function, and kidney failure. Therefore, urethral strictures are serious health conditions that significantly impair quality of life and may lead to the failure of vital organs.3
Other pathologies that require the use of grafts for the reconstruction of the urethra are epispadias and hypospadias in pediatric patients. Treatment of strictures usually begins using less invasive techniques, such as urethral dilatation and optical urethrotomy. The disadvantage of these procedures is a common recurrence of strictures. Therefore, when restrictures occur or larger sections of the urethra are strictured, other invasive procedures such as urethral resection followed by anastomosis, multi-procedure urethral plastics, buccal or lingual autologous transplants, respectively, are carried out.4
Despite the vast experience, urethral reconstruction continues to be a challenging field for urologists. While for some conditions, only one or few procedures are standard, over 300 techniques are known for urethral stricture and hypospadias repair.5 For example, Steenkamp et al.6 indicated that the success of (optical) urethrotomy for a year’s follow-up, given that the stricture was less than 2 cm, was 60%, but only 20% if the stricture was longer than 4 cm. Another group showed that the subsequent (second) urethrotomy leads to improvement in only 40% of subjects in 48 months’ follow-up.7 However, if restrictures occurred earlier (<3 months), the rate of “failure” that led to the invasive procedures was 100% for the period of 48 months. Resection of urethral stricture followed by reanastomosis is an effective surgical method with a success rate approaching 90%, but only for so-called frontal urethral strictures. These operations are technically feasible only for short strictures.8 Longer strictures require the use of skin flaps or graft to fill out the defect. Dubey et al.9 have shown that there is no difference in success rate in the use of skin flap or buccal mucosa graft, but the use of skin flap led to greater morbidity. Therefore, buccal mucosa grafts are the preferred choice for substitution of missing biological material. The oral mucosa has become the graft of choice because of its availability.10
The use of buccal mucosa graft is also limited due to several reasons. Patients with limited mouth opening or by prior oral surgery may have a smaller area of tissue that is suitable for use. In addition, the collection of larger grafts is associated with greater morbidity. Complications associated with the collection of oral mucosa grafts include intraoperative bleeding, postoperative infection, pain, swelling, damage to the salivary ducts, mouth opening limitation, altered sensitivity or insensitivity, and scar deformities. The risk of complications after collection graft is increased in smokers and in patients with poor oral hygiene.11
As described above, all of these procedures and approaches for urethral reconstruction have limitations compared to the autologous urethral tissue. Therefore, tissue engineering (TE) applying different types of cells, biomaterials, and specific growth factors to produce various anatomical structures, including the urethra, may contribute to overcome these problems.12
This review article aims to present a summary of recent literature on tissue engineering and its implication for urethral reconstruction. The selected studies are divided into three main categories: autologous cells, scaffolds, and clinical results.
Methods
Literature search
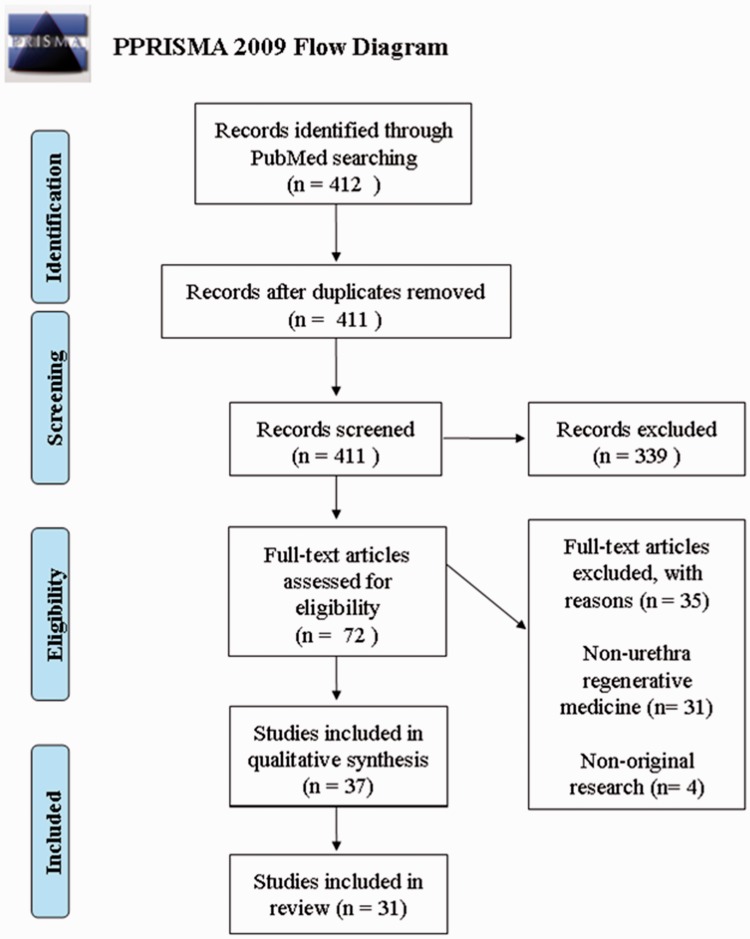
A search was performed (18 January 2017) in the databases of PubMed/Medline. Key words related to tissue engineering (e.g. stem cells, autologous grafts, scaffolds, etc.) were combined with synonyms for the urethra and urothelial tissue. The search was restricted to the last five years, the English language, and studies performed on humans or animals. Prisma Flow Diagram represents the outline of the literature search (Figure 1).
Figure 1.
Outline of the literature search (n = 31). The database search was performed on 18.1.2017 according to the PRISMA statement. For more details see Method section. (A color version of this figure is available in the online journal.)
Study selection
The obtained results of the search from databases were manually checked and duplicates were removed. The title screening was done by author MG. The abstract screening was performed independently by MG and LD. The obtained screening results were compared and any differences were resolved by discussion. Full-text screening was performed by SZ with the assistance of MG. All studies dealing with tissue engineering of urethra were included. All papers in languages other than English were excluded.
Data extraction and analysis
From the selected studies, the following data were extracted. For the cell section: cell type, scaffold, the number of passages, culture, and results. For the scaffold section: cell type, scaffold type, the aim of the study, and results. For the clinical results section: number of patients, cell type, scaffold, urethral pathology, follow-up, and results. The data were imported in MS Office Excel 2010 database.
Results
Cells
Table 1 presents the details of 10 studies describing the use and the culture of various cells in the context of urethral TE. The main focus was oriented on adipose-derived stem cells, urine-derived stem cells, human urothelial cells, human umbilical cord mesenchymal stem cells, and keratinocytes.
Table 1.
Overview of studies describing the use and the culture of various cells in the context of urethral TE
| References | Cell type | Scaffold | Number of passage used for experiment | Culture (weeks) | Results |
|---|---|---|---|---|---|
| Shuai et al.13 | human umbilical cord-derived mesenchymal stromal cells | No | 3 to 6 | 2 | Differentiation of hUCMSCs into urothelial cells in vitro: morphology of hUCMSCs changed from spindle-shape to a polygonal epithelial shape; expression of CK18 and UPII observed in the culture of hUCMSCs in UC-CM+ exogenous EGF. |
| Li et al.14 | autologous rabbit oral keratinocytes, transforming growth factor-ß1 small interfering RNA-transfected fibroblasts | Bladder acellular matrix graft | 2 (oral keratinocytes) | 1 | tissue engineered mucosa successfully repaired urethral defect in animal model, no signs of stricture or fistula observed |
| Lang et al.15 | Urine-derived stem cells | No | 2 | 3 | defining USCs after preservation in urine for 24 h: rice grain shape in primary culture, expressed surface markers characteristic for mesenchymal stem cells and displayed bipotent differentiation capability, urothelial cell-specific markers expressed when exposed to urothelial differentiation medium |
| Sun et al.16 | Hypoxia-activated human umbilical cord mesenchymal stem cells, skeletal muscle cells (rabbit) | No | – | 3 | vessel structures and muscle fibers were observed in engineered, pre-incubated constructs after three weeks; constructs used as grafts for urethral reconstruction could repair defected urethras without developing stricture, fistula or pseudo-diverticulum |
| Chun et al.17 | Autologous healthy urethral muscle and endothelial tissue | Porcine bladder acellular matrix | – | – | tissue/scaffold construct could anatomically and histologically repair defected urethra, thick urothelial layer, circular bundles of smooth muscle and vascularization were present in the neo-urethral tissue |
| Wang et al.18 | Adipose-derived stem cells (canine) | Polyglycolic acid | 1 | Induction of canine adipose-derived stem cells by 5-azacytidine performed for four weeks; cell-scaffold construct cultivated statically for one week and for five weeks in a bioreactor | Adipose-derived stem cells acquired myoblast phenotype; muscular tube successfully engineered |
| Zhang et al.19 | Human adipose-derived stem cells | No | 3 | Induction using conditioned medium or indirect co-culture methods; culture lasted for 1–3 | human adipose-derived stem cells demonstrated the potential to differentiate towards urothelium-like cells |
| Li et al.20 | Epithelial-differentiated rabbit adipose-derived stem cells | bladder acellular matrix graft | 3 | Adipose-derived stem cells were induced in epithelial-specific culture system for 12 days; cell-scaffold construct cultivated for a week | Epithelial-differentiated rabbit adipose-derived stem cells differentiated into urothelium |
| Kang et al.21 | Urine stem cells, adipose-derived stem cells (human) | No | 3, 5, 7 | Incubation lasted for two weeks, in vitro multi-lineage differentiation: culture terminated at day 7 for neuron, day 14 for adipocyte,osteoblast and myocyte, day 21 for endothelium and chondrocyte | Morphology differences: urine-derived stem cells had cobble stone-like shape, adipose-derived stem cells had spindle-shaped morphology; analysis of colony formation and multi-lineage differentiation showed better results for urine-derived stem cells |
| Rogovaya et al.22 | Autologous epidermal rabbit-labeled keratinocytes | – | – | – | graft composed of living skin equivalent with labeled keratinocytes could repair damaged urothelium |
TE: tissue engineering
One study described the technique of engineering the urethral muscular tubes in a bioreactor. The polyglycolic mesh was seeded with canine adipose-derived stem cells. Prior to creating cell-scaffold complex, cells had been induced by 5-azacytidine in order to acquire a myoblast phenotype. The static culture of cell-seeded complex lasted for one week. Thereafter, analysis using scanning electron microscopy was performed on some samples. Others were transferred to a bioreactor and subjected to extension simulation for five weeks. Microscopic observation and immunofluorescence analyses determined the differentiation of adipose-derived stem cells towards myoblasts. The muscular tube was engineered using undifferentiated canine adipose-derived stem cells and polyglycolic mesh after five weeks of incubation in a bioreactor system.18
Differentiation ability of human adipose-derived stem cells towards urothelium was investigated in a study carried out by Zhang et al.19 Immortalized cell line from the human urinary bladder (HUC) was used for the induction experiments. Cells were indirectly co-cultured with HUC-s or induced by HUC-derived conditioned media. Culture lasted for one to three weeks. Polygonal morphology and rich in the cytoplasm were observed after three weeks’ induction. Cobblestone-shaped morphology typical for urothelium was not detected. Adipose-derived stem cells expressed specific markers uroplakin-IA and uroplakin-II when indirectly co-cultured through a transwell system and also when cultured with conditioned media.
Li et al.20 studied the potential of epithelial-differentiated rabbit adipose-derived stem cells seeded on bladder acellular matrix grafts in order to repair the anterior urethral defect. Culture was in epithelial induction system which imitated epithelial-specific microenvironment. After the differentiation, epithelial-differentiated rabbit adipose-derived stem cells were used for the in vivo study. Analysis of induced cells demonstrated their epithelial-like morphology and expression of cytokeratin 19 and 13. Thirty-six rabbits were used for the experiment and divided into three equal groups. Non-seeded bladder acellular matrix graft was applied in substitution urethroplasty in control group 1. Animals in control group 2 underwent urethral reconstruction with the use of undifferentiated adipose-derived stem cells seeded on the scaffold. Study group consisted of 12 rabbits which received the engineered graft composed of epithelial-differentiated adipose-derived stem cells seeded on bladder acellular matrix. Surgical outcomes showed a development of postoperative complications in control groups. Retrograde urethrograms were performed and revealed severe strictures, excessive contractures, and fibrosis in both control groups two weeks and one month after urethroplasty. Mild urethral stricture and contracture were observed in a study group at two weeks after surgery. However, these findings were markedly improved at 1 month post-implantation. Analyses of the grafts were performed at one week of in vitro culture using transmission electron microscopy. Both undifferentiated and epithelial-differentiated rabbit adipose-derived stem cells formed a stratified structure on bladder acellular matrix.
One study investigated the safety of the urine-derived stem cells’ preservation in the urine for 24 h. Four hundred fifteen urine samples were collected from 12 healthy men. Firstly, a total number of cells shed into the urine (urine-derived cells) during 24 h were determined; 189 samples were subsequently preserved in several preservation solutions and the effect of the preservation was evaluated by considering survival and function of urine-derived stem cells. Fresh and 24 h preserved samples were compared. According to the results, preservation did not have any impact on the cells’ quality, as their shape, characteristic surface markers, and bipotent differentiation capability (myogenic and urothelial) typical for mesenchymal stem cells were still present and after their exposition to urothelial differentiation medium, uroplakin 1A, uroplakin III, and cytokeratins 13, 7, and 20 were detected.15
Selecting the appropriate cell source for clinical application is crucial. The characteristics of urine-derived stem cells and adipose-derived stem cells were compared in study realized by Kang et al.21 Proliferation, immune modulation, and multi-differentiation were evaluated for both cell types as well. Urethral catheterization was performed in order to obtain urine samples from which were the urine-derived stem cells isolated. Human adipose tissue was obtained for the isolation of adipose-derived stem cells. Thereafter, both types of cells were cultured and passages number 3, 5, and 7 were used for analysis. Morphology analysis showed differences between urine- and adipose-derived stem cells. While urine-derived stem cells had cobble stone-like shape with frill, fibroblast-like shape presented adipose-derived stem cells. The higher proliferative capacity of both cell types was demonstrated on early cell passage number and urine-derived stem cells showed increased proliferation rate at all cell passages. Better results of colony formation were observed in urine-derived stem cells, as well. Compared to adipose-derived stem cells, analysis of multi-lineage differentiation (myogenic, neurogenic and endothelial) showed better results for urine-derived stem cells.
The use of autologous urethral tissue for the urethroplasty was investigated in a study carried out by Chun et al.17 Autologous urethral tissue was minced and placed onto acellular bladder submucosa matrix. In this animal model study, 20 rabbits were divided into four following groups: normal control, urethral stricture, rabbits with only bladder submucosa matrix application, and rabbits with tissue-scaffold construct application. Stricture of the long urethral segment was created and after four weeks, animals assigned to graft groups underwent the operation. Retrograde urethrography was performed at 4, 8, and 12 weeks post-operatively. Animals were sacrificed three months after surgery, and histologic and immunohistochemical analyses were performed. In the group, where healthy autologous urethral muscle and endothelial tissues were used, multi-layered stratified columnar epithelium lined the neo-urethral lumen, bundles of smooth muscle, and neovascularization was present. Compared to the group, in which only bladder submucosa matrix was used, fibrotic changes, low muscle distribution, and keratinized squamous epithelium were detected. The authors of the study claimed that rapid urethral regeneration was stimulated by using autologous urethral tissue as a cell source.
The potential of human umbilical cord-derived mesenchymal stromal cells was investigated in two studies. Sun et al.16 engineered a pre-vascular construct composed of pedicled muscle flaps and hypoxia-activated human umbilical cord-derived mesenchymal stem cells suitable for urethral reconstruction. In this research, 28 New Zealand rabbits were involved: 21 belonged to the experimental group and 7 to the control group. Rabbits underwent biopsy of skeletal muscle. Harvested samples were subsequently minced and fragments were mixed with hypoxia-activated human umbilical cord mesenchymal stem cells. This mixture was then injected into the rabbits’ ventral penile subcutaneous cavity to create a construct suitable for urethral reconstruction. Pre-incubation lasted for three weeks. However, the mixture of umbilical cord-derived stem cells and minced muscle was used only in the experimental group. Control group received only the implant of minced muscle. In the experimental group, pre-incubated constructs were implanted into damaged urethras. No construct was formed in the control group, so the skeletal muscle patch was used in the urethral reconstruction. Results in experimental group demonstrated the presence of vessel structures. Moreover, grafted urethras were covered with urothelium, had wide caliber, and no signs of obvious stricture or fistula were observed.
Another study concerned the human umbilical cord-derived stem cells as a source for urethral tissue engineering, as well. The aim was to establish their urothelial differentiation. Three experimental groups were designed for the in vitro study. Human umbilical cord-derived mesenchymal stem cells were cultured with conditioned medium from urothelial cells (UC-CM), or with UC-CM supplemented with exogenous epidermal growth factor (EGF) using its different concentrations. In the last group, Dulbecco’s modified Eagle medium (DMEM) together with EGF was used for culture. Results showed the best conditions for urothelial differentiation provided the culture of hUCMSCs in UC-CM together with 20 ng/ml exogenous EGF. Expression of uroplakin II and cytokeratins was present, the morphology of stem cells changed into epithelial-shape after week.13
Oral keratinocytes were in the interest of two studies. In one of these were seeded on bladder acellular matrix together with transforming growth factor ß (TGF-ß) siRNA-transfected fibroblasts in order to create tissue-engineered mucosa. This graft was subsequently used for urethral reconstruction in the animal model. Twenty-seven male rabbits were involved in the study and divided into three groups of nine animals in each. The first group received grafts consisting of autologous oral keratinocytes and TGF-ß1 siRNA-transfected fibroblasts seeded on bladder acellular matrix. Autologous oral keratinocytes alone seeded on matrix were used in group 2 and unseeded grafts were applied in group 3. Retrograde urethrograms were performed to assess urethral caliber at one, two and six months postimplantation. Results of in vitro study showed good biocompatibility of both types of cells with bladder acellular matrix. In vivo outcomes revealed wide urethral caliber, no signs of stricture or fibrosis in groups 1 and 2. Histological analyses after six months showed stratified epithelial layer formation in groups 1 and 2. However, the formation of capillary structures was observed only in the group 1.14
Living skin equivalent (LSE) based on autologous rabbit keratinocytes and fibroblasts was used to repair the urethral injury in an animal model. LSE with labeled keratinocytes was implanted into the de-epithelialized urethra and the behavior of the keratinocytes was analyzed. Seventeen chinchilla rabbits underwent surgery using different types of grafts. The experimental group (n = 11) was given LSE with labeled keratinocytes, and two control groups (n = 6) consisted of animals receiving no transplant or cell-free Spongostan gelatin sponge. All animals belonging to control group developed complications within two weeks after the surgery. Results obtained from the experimental group showed complete recovery of urethral epithelium and urethral function, as well.22
Scaffolds
According to our literature search, 19 studies described the scaffold approach in terms of urethral reconstruction (Table 2).
Table 2.
Overview of studies describing the use of various scaffolds in the context of urethral TE
| References | Cell type | Scaffold type | Aim of the study | Results |
|---|---|---|---|---|
| Sartoneva et al.23 | Human urothelial cells | Smooth PLCL, textured PLCL, compression-molded PLCL | To compare different types of PLCL-membranes for optimizing the growth surface for human urothelial cells | Both smooth and textured PLCL significantly supported the cells' growth |
| Micol et al.24 | Autologous rabbit smooth muscle cells | Acellular or cell-seeded collagen gel tubes | To engineer high-density collagen gel tubes serving as urethral grafts in male New Zealand rabbits (rabbit model) | High-density collagen gel tubes regenerated a rabbit urethral segment by spontaneous regrowth of urothelium; no signs of inflammation were present |
| Sayeg et al.25 | Autologous rabbit smooth muscle cells | Acellular or cell-seeded natural heterologous collagen matrices | To evaluate the integration of the matrices into the rabbits' urethras when implanted with no cells or as cell-seeded patches (rabbit model) | The incorporation of the matrices into urethral walls was not present, but both types of matrices (seeded or acellular) were able to restore cell architecture of the urethra |
| Orabi et al.26 | Autologous canine bladder epithelial and smooth muscle cells | Tubularized collagen-based matrices (derived from the bladder) | To engineer cell-seeded tubularized scaffold suitable for long urethral defects reconstruction (canine model) | Cell seeding on the acellular collagen matrix using autologous cells resulted in the development of normal urethral tissue layers |
| Martín-Cano et al.27 | – | Rat oral mucosa | To evaluate histological and functional changes in rat oral mucosa when implanted as a graft in the rat urethra (rat model) | Study confirmed clinical usefulness of oral mucosa as a graft for urethral reconstruction; oral mucosa manifested significant histological changes by forming well-developed epithelium; no local complications were present |
| Xie et al.28 | Canine urothelial cells | Stretched electrospun silk fibroin matrices | To evaluate the use of the stretched electrospun silk fibroin matrices in urethral reconstruction (canine model) | Matrices showed good biocompatibility and supported the growth of urothelial cells; when implanted as a tissue-engineered mucosa, canines voided without any difficulties and no signs of strictures were present |
| Chung et al.29 | – | Bi-layer silk fibroin scaffolds | To investigate characteristics of this biomaterial in order to support tissue regeneration; scaffolds compared with small intestinal submucosa implants (rabbit model) | Scaffolds capable of tissue regeneration, no signs of chronic inflammatory reaction three months post-operation |
| Jerman et al.30 | Porcine urothelial cells | Amniotic membrane epithelium, denuded amniotic membrane, stromal amniotic membrane | To assess the formation of urothelium on different types of amniotic membranes | Urothelium was developed on all types of amniotic membrane scaffolds. However, stromal amniotic membrane demonstrated the fastest cells' growth and highest cells' differentiation |
| Wang et al.31 | Rabbit urethral epithelial cells | Cell-seeded or acellular denuded human amniotic membrane | To maximize the biocompatibility and minimize rejection of human amniotic membrane when used as a scaffold by separating of the basement layer and obtaining denuded human amniotic scaffold (rabbit model) | Cell-seeded denuded human amniotic scaffold showed good potential for the use in urethral reconstruction |
| Wei et al.32 | Rabbit oral mucosal epithelial cells | PCL/silk fibroin/collagen electrospun nanofiber scaffold | Preparation of PCL/silk fibroin/collagen scaffold and evaluate its effects on cells' growth and proliferation | PCL/silk fibroin/collagen scaffold demonstrated good cell growth, cell compatibility, appropriate pore size and porosity and was expected to become a suitable scaffold for urethral reconstruction |
| Kajbafzadeh et al.33 | – | Fibrin sealant, preputial acellular matrix | To compare several techniques for segmental urethral reconstruction (rabbit model) | The use of preputial acellular matrix with or without the application of the fibrin glue for the urethroplasty showed satisfactory results – wide urethral caliber and no signs of strictures were present |
| Heller et al.34 | Primary buccal epithelial cells, fibroblasts, microvascular endothelial cells | Native and cross-linked collagen membranes | To engineer pre-vascularized buccal mucosa equivalents | Pre-vascularized buccal mucosa equivalents were successfully engineered using a triculture of buccal epithelial cells, fibroblasts and microvascular endothelial cells; compared to cross-linked collagen membrane, the higher formation of capillary-like structures were found on native collagen membrane |
| Imani et al.35 | Normal porcine urothelial cells | Nanostructured titanium dioxide (TiO2) scaffolds: TiO2 nanotubes, nanowires, nanospheres | To create TiO2 scaffolds using the anodization method to synthetize TiO2 nanotubes and chemical vapor deposition method to synthetize TiO2 nanowires and nanospheres | TiO2 nanowires showed the best potential for urologic applications in tissue engineering |
| Jia et al.36 | – | Collagen scaffolds modified with collagen-binding VEGF | To investigate, whether this type of collagen scaffold could enhance regeneration of long urethral defects | Urethral tissue regeneration was observed |
| Zhang et al.37 | Rabbit bladder epithelial cells and dermal fibroblasts | Wnt pathway inhibitor delivering collagen/poly(L-lactide-co-caprolactone) scaffold | To evaluate mechanical properties and biocompatibility of the scaffold as well as evaluating the potential of inhibiting extracellular matrix expression in vitro and in vivo (rabbit model) | Collagen/P(LLA-CL) scaffold loaded with Wnt pathway inhibitor could facilitate the decrease of fibroblasts' deposition in the ECM; scaffold delivering ICG-001 had significant anti-fibrosis effects and good biocompatibility and mechanical strength |
| Lv et al.38 | Human a mniotic mesenchymal stem cells | Poly(L-lactide)/Poly(ethylene glycol) | To assess the potential of combining two biodegradable polymers (PLLA/PEG) to create a scaffold and seed it with hAMSCs for urethral epitelium repair (rabbit model) | Best result was observed in the group of rabbits where seeded PLLA/PEG scaffold had been used for the repair of the urethral defect |
| Lv et al.39 | – | Oxygenating keratin/silk fibroin scaffold | To evaluate properties and impact on urethral regeneration of the new type of scaffold that was able to release high level of oxygen | Oxygen-delivering scaffold supported cell growth; when implanted in the rabbits, intact epithelial layer and bundles of muscle were present |
| Aufderklamm et al.40 | Human urothelial cells | bovine collagen type I-based cell carriers | To evaluate the use of cell-seeded bovine collagen cell carrier and outcome of the surgical procedure (minipig model) | Bovine collagen cell carriers seeded with human urothelial cells used as xenografts showed technical feasibility |
| Pinnagoda et al.41 | – | Acellular high-density collagen tube | To engineer tubular collagen scaffold and apply it as an urethral graft (rabbit model) | Evaluation of the scaffolds revealed excellent biocompatibility and suture suitability. When applied in vivo, 40% of the animals developed complications |
TE: tissue engineering
A study carried out by Sartoneva et al.23 investigated different PLCL-based membranes aiming to optimize the mechanical characteristics and scaffold’s surface for cell growth. In vitro degradation was also investigated. Membranes consisting of smooth, textured PLCL and compression molded PLCL were compared. Hydrolysis of membranes was carried out, and membranes were also tested for tensility. Human urothelial cells (obtained from normal ureters of child donors) were seeded onto each membrane, and scanning electron microscopy was used to evaluate cells’ attachment and morphology after 2 h, 7 and 14 days of culturing. Cytokeratin 7 and 19 were used for immunostaining. Results showed that best mechanical properties and in vitro degradation had textured PLCL. Furthermore, textured PLCL together with smooth PLCL supported the attachment and proliferation of human urothelial cells.
The use of collagen-based membranes is described in subsequent studies.
High-density collagen gel tubes were engineered and used as acellular or seeded tissue-engineered grafts for urethral repair in New Zealand male rabbits. Sixteen animals were included in this study. After the rabbits underwent the bladder biopsy, rabbits’ smooth muscle cells were obtained and cultured separately for each rabbit for one month. The critical-sized iatrogenic urethral defect was created and the acellular or seeded graft was implanted into the urethra. Grafts’ parameters were 2 cm in length and internal diameter was 3 mm. Control radiography was performed and animals were sacrificed one or three months after the surgery. Analysis of urethrograms revealed that 5 rabbits had normal urethrograms and remaining 11 animals developed partial narrowing of urethra (n = 7). Isolated fistulae were found in two cases and three times were associated with urethral narrowing. Complete stenosis associated with fistulae was found in two rabbits. Fewest complications and satisfactory lumen calibers were detected in the group, where seeded high-density collagen gel tubes were used for the urethral reconstruction.24
Another animal model study used decellularized heterologous collagen matrices as onlay grafts for urethral implantation. Eighteen New Zealand rabbits were randomly divided into two equal groups. Group 1 underwent surgery where a patch of heterologous collagen matrix was seeded with autologous smooth muscle cells and implanted into the urethra. The second group underwent a surgery, where resected urethral segment was replaced only with decellularized heterologous collagen matrix patch. Collagen matrices were obtained from porcine bladders submucosa. Performed urethroscopies and cystourethrografies showed urethras with the normal caliber and good urinary flow. Histological analyses were performed at one, two, three weeks and three months postoperatively on both acellular and cell-seeded matrices. The entire urethral architecture was restored in both groups three months after the operation. However, less intense inflammatory process was present at this time, as well. Results of this study demonstrated that cell-seeded or acellular heterologous collagen matrices could restore the entire cell architecture of the urethra, but the grafts were not incorporated into its walls.25
Orabi et al.26 carried out a preclinical study where collagen-based tubular matrices were used for the reconstruction of long urethral defects. Twenty-one male dogs had their urethral segments removed and replaced with cell-seeded or acellular tubular matrices. Autologous bladder epithelial cells and smooth muscle cells were harvested from 15 male dogs. Decellularized bladder matrix was tubularized around the sterile urethral catheter and sutured. The internal surface of the scaffold was statically seeded with urothelial cells. Muscle cells were statically seeded onto the outer side of the scaffold. Fifteen dogs underwent urethroplasty with seeded scaffolds (experimental group) and six animals received only acellular tubular constructs (control group). The length of transected and removed urethral segment was 6 cm. Bladder catheter was surgically implanted and left in the bladder for four weeks postoperatively. For the follow-up, serial urethrographies and three-dimensional computed tomographies were performed at 1, 3, 6 and 12 months postoperatively. Animals were humanly euthanized for analyses. The results showed that strictures, fibrosis, and fistulae developed in the control group. Patent urethra with normal caliber was present in all animals belonging to experimental group.
In a study carried out by Jia et al.,36 collagen scaffold was modified with collagen binding VEGF. This protein seemed to improve scaffold’s properties, as it stimulated cell proliferation and angiogenesis. Modified scaffolds were used for the repair of the long urethral defect in canine model. Ten beagles were assigned to two groups: one group in which unmodified tubularized scaffolds were applied, and the other group where tubularized modified scaffolds were used. All animals with unmodified scaffold delivered following post-operative symptoms: dysuria, incomplete emptying, poor urine stream, fistulas were observed in three beagles. Two animals underwent cystostomy due to severe dysuria. Opposite to this group, animals belonging to the other one did not develop such post-operative complications. Mild dysuria was observed in one animal, and severe dysuria was detected in two animals that also underwent cystostomy. Two dogs in this group did not develop any complications. Strictures were present in both groups; however, more severe detected in the unmodified scaffold group. Histological analysis revealed that all animals had the collagen biomaterial degraded at six moths post-operation. Moreover, neourethra was fully covered with 8–10 epithelial layers in four beagles in modified scaffold group. In this group, significantly higher revascularization was observed, and more smooth muscle bundles were detected as well. However, muscle bundles appearing in the repaired area did not have the good organization in both groups.
Rat-tail collagen was used for the acellular collagen scaffold engineering in a study carried out by Pinnagoda et al.41 High-density collagen tubes consisting of two layers were generated and applied in vivo. The advantage of this double-layered collagen gel tube was a good surgical handling, there was no need for synthetic polymer support. Iatrogenic 2 cm long urethral defect was created in 20 rabbits. Collagen gel tubes were implanted and sutured to the native urethra. Animals were examined at one, three, six and nine months post-operatively. Results of voiding cysto-urethrography showed that 40% of animals developed complications: distal fistulas observed in 20% of the rabbits, secondary fistulas associated with distal stenosis detected in 20% of the animals. During nine months’ period, gradual increase of urothelial cells and their subsequent stratification was observed. Vascularization was detected at one month and was gradually developing. Muscle cells were firstly detected at three months and bundle formation was present at six months. After nine months, time-dependent urethral regeneration using double-layered collagen gel tubes was observed.
Adequate vascularization of tissue-engineered transplants is a crucial, however, limiting factor. Native collagen scaffold was used for engineering of pre-vascularized buccal mucosa equivalent and compared with highly cross-linked collagen membrane in terms of the formation of the capillary-like structures. Engineering of buccal mucosa equivalent consisted of generating the triculture of cells seeded onto the scaffold. Epithelial cells and fibroblasts were obtained from human gingiva, and microvascular endothelial cells were harvested from the human juvenile foreskin. Triculture was generated subsequently. Firstly, the endothelial cells were seeded onto the rough side of the collagen scaffold and incubated for 24 h. After this incubation, fibroblasts were added on the top and endothelial cells were incubated together with fibroblasts for another three days. Collagen membrane was then carefully turned upside down and epithelial cells were seeded. Analysis of the scaffold was performed after three weeks of the cultivation. The result showed that poor formation of capillary-like structures was found on cross-linked collagen membrane. On the other hand, native collagen membrane demonstrated the formation of the epithelial layer on the upper side of the membrane with a deep infiltration of the fibroblasts from the bottom side. Furthermore, successful pre-vascularization and superficial capillary-like structures were manifested, as well.34
In another study, where rat oral mucosa was used as an autologous graft, 26 animals underwent urethroplasty and histological changes of the implants were analyzed. Results showed well-developed epithelium with no presence of complications and demonstrated the suitability of this tissue for urethral reconstruction.27
Collagen cell carriers were used in xenograft minipig study design. The aim of this pilot study was to determine whether cell-seeded bovine collagen cell carriers could be implanted as a graft in the animal model. Human urothelial cells were obtained from the benign ureteral tissue samples. Four male Göttingen minipigs with immunosuppression were included in the study. Two surgical interventions were performed on the animals. In the first one, iatrogenic urethral stricture was induced by monopolar thermocoagulation. Protective vesicostomy was performed to guarantee continuous and free urine flow and good wound healing of the penile urethra. Seven weeks after stricture induction, ventral onlay urethroplasty with stratified cell-seeded collagen carriers was performed. Strictured part of the urethra was opened, and grafts were applied luminal in two minipigs, and anti-luminal in other two animals. Final urethrography revealed no signs of the reappearance of the strictures in all four urethras. Despite complete histological integrity was found in all urethras, paravasation was observed in one minipig.40
A study carried out by Kajbafzadeh et al.33 aimed to evaluate the potential of fibrin sealant and preputial acellular matrix as a new source of the collagen matrix. Several techniques of segmental urethral reconstruction were compared in the animal model. Twenty-four male New Zealand rabbits were chosen for this study and randomly divided into four groups. Urethrotomy was closed in layers in groups 1 and 2, but this procedure was subsequently followed by injecting the fibrin sealant over the suture lines in group 2. In two other groups, the preputial matrix was used as a graft for urethral reconstruction and application of fibrin sealant was added in group 4. Urethral tissue was analyzed one, three and nine months postoperatively. Best results were obtained in the groups, where preputial acellular matrix with or without fibrin sealant was used for the urethral repair.
The growth and proliferation of oral mucosal epithelial cells were tested on scaffold composed of polycaprolactone/silk fibroin/collagen nanofibers. The electrostatic spinning method was used to prepare this nano 3D porous scaffold. Oral mucosal tissue was obtained from 10-week-old male rabbit and used for the isolation of oral mucosal cells, which were seeded onto the scaffold. The cell-scaffold composite was cultivated for five days and subsequently analyzed. Scanning electron microscopy revealed that scaffold’s nanofibers had a smooth surface, uniform diameter, and cells were tightly attached to this surface. Cell growth was satisfactory, as well, which demonstrated good compatibility and potential use in urethral reconstruction.32
Lv et al.39 evaluated the potential of keratin/silk fibroin oxygen releasing scaffold for urethral tissue engineering. Keratin substance was extracted from human hair. Gelatin and calcium peroxide were incorporated into the keratin-silk compound. Cytotoxicity and oxygen generation were evaluated. For in vivo testing, the animal model was used and scaffolds with and without calcium peroxide were implanted into rabbits’ urethras. For the cytotoxicity and proliferation testing, rabbit smooth muscle cells were seeded onto scaffolds and cultured for two weeks. In vivo testing included 18 New Zealand rabbits assigned to three groups: A- experimental, B- scaffolds without calcium peroxide used, and C-small intestinal submucosa applied. The ventral urethral defect with the length of 1.5 cm was created and animals underwent the surgical procedure. After the six months’ period, the surgical outcome was evaluated. Analysis of the cytotoxicity revealed that smooth muscle cells were more viable on oxygen realizing scaffold. Moreover, cells created a thicker layer on this type of scaffold as well. In vivo evaluation showed that all rabbits in the group B had a fistula. Less epithelium and more inflammatory cells together with fibroblasts were also observed. Patent urethral caliber was detected in groups A, C, as well as compact epithelial layer and several muscle bundles. This study demonstrated that generating high oxygen levels could significantly promote growth and proliferation of the cells. The antibacterial ability of this scaffold was manifested as well.
Another study described the use of biodegradable poly(L-lactide)/poly(ethylene glycol) (PLLA/PEG) scaffolds seeded with human amniotic mesenchymal stem cells to repair 2 cm-long urethral defects in New Zealand rabbits. Electrospinning technique was used to prepare scaffolds. Mechanical properties were evaluated by tensile testing, and biocompatibility was examined using Cell Counting Kit-8 assays. Twenty-seven male New Zealand rabbits were included in this study and divided into three groups (A, B, C). Animals underwent surgeries, where artificial urethral defects were repaired by several techniques. In group A, a cell-seeded scaffold was used for the repair. Acellular PLLA/PEG scaffold was used in group B and regular urethral reparation technique was applied in group C. Retrograde urethrograms were performed to evaluate urethral tissue before and after the sacrifices. The best repair of the urethral defects was observed in group A.38
One study demonstrated that acellular bi-layer silk fibroin scaffolds could enhance tissue regeneration of the damaged urethra. Moreover, scaffolds had reduced immunogenicity as well. In this animal model study, male rabbits were divided into three groups. Different grafts were implanted in mentioned groups: group 1 received silk fibroin grafts (experimental group), group 2 small intestinal submucosa implants and urethrotomy alone was performed in the third group. Animals assigned to experimental group developed only minimal inflammatory response detected three months post-operatively. In addition, the urethral defect was thoroughly regenerated.29
Stretched electrospun silk fibroin matrices were investigated in terms of potential use for urethral reconstruction in a study carried out by Xie et al.28 Scanning electron microscopy and a porosity test assessed material's structure and characteristics. Scaffolds were seeded with canine urothelial cells, and after one week of cultivation, the tissue-engineered graft was created and implanted in six female beagle dogs. Prior to the implantation, dorsal urethral mucosal defect had been created. Control group consisted of three dogs, in which no substitute was used for the urethral reconstruction. Retrograde urethrography performed at one, two and six months postoperatively revealed wide urethral caliber with no signs of stricture in the experimental group. Moreover, canine urothelial cells (obtained from canine bladders’ biopsy) covered the defect forming stratified layers, which were observed six months post-operatively. Complications such as severe inflammation and urethral stricture developed in the control group.
Other types of scaffolds and their potential for urologic applications were investigated in a study carried out by Imani et al.35 Anodization method was used to synthesize TiO2 nanotubes and chemical vapor deposition technique was applied for TiO2 nanowires and nanospheres generation. Scanning electron microscopy and X-ray diffraction methods were used for the scaffold analysis. Different nanostructured TiO2 scaffolds were seeded with normal porcine urothelial cells. In the control group, the standard porous membrane was seeded with these cells. After three weeks of cultivation, analysis revealed that normal porcine urothelial cells were viable, tightly attached, and grew well on all membranes. However, largest cells were found on TiO2 nanowires, while the smallest were on the porous membrane. This study demonstrated the promising use of TiO2 scaffolds, especially TiO2 nanowires, in urologic applications.
Inhibition of the urethral fibrosis still remains challenging. The aim of the study, where Wnt pathway inhibitor delivering scaffold was prepared, was to evaluate biocompatibility and mechanical properties of this construct. Inhibition of extracellular matrix expression in vitro and in vivo was analyzed, as well. The main compound of the scaffold was collagen/poly(L-lactide-co-caprolactonate) and co-axial electrospinning technique was used to load the Wnt pathway inhibitor into the scaffold structure. Non-drug scaffolds were also used in the study. Bladder and dermal biopsy were performed in order to obtain epithelial cells and fibroblasts. Each scaffold was seeded with bladder epithelial cells and cultivation took one week. These structures were then implanted into the animals. Cultivation and passaging of the fibroblasts served for anti-fibrosis testing. Twelve rabbits were included in the study, randomly divided into two equal groups and underwent the surgical procedure. According to a specified group, rabbits underwent urethroplasty of a 2 cm long defect. Drug delivering scaffold seeded with cells was applied in group 2 and the results demonstrated no strictures or fistulas. Moreover, wide urethral caliber was present. Urethrography performed in group 1, where non-drug delivering, cell-seeded scaffold was used, showed complications such as narrowing of the urethral lumen together with the strictures and fistulas. Expression of collagen type 1, 3 and fibronectin of the fibroblasts that were treated with the medium released from drug delivering scaffolds could be inhibited at mRNA and protein levels.37
Two studies evaluated the use of a human amniotic membrane as a scaffold for the urethral reconstruction. One of these studies investigated whether denudation of human amniotic membrane could suppress the immune response and thus enhance its biocompatibility. Human denuded amniotic membrane was seeded with rabbit urethral epithelial cells or remained acellular. Twenty male New Zealand rabbits were included in this study and divided into following groups. Subcutaneous implantation of the human denuded or human amniotic membrane was performed on eight rabbits. Other 12 animals underwent urethral reconstruction. Tissue-engineered denuded human amniotic membrane was transplanted in six animals (experimental group). The remaining rabbits formed a control group, where the urethral defect was repaired by using intact denuded human amniotic membrane patch. Results showed good histocompatibility of the denuded human amniotic membrane at eight weeks after subcutaneous implantation. No serious inflammation or rejection was observed. The outcome of the surgical procedures revealed that no inflammation or fistula was found in the experimental group. However, inflammation and fistula developed in one rabbit belonging to the control group.31
The second study investigated the use of the amniotic membrane, as well. In this one, normal porcine urothelial cells were seeded on the amniotic membrane epithelium, denuded amniotic membrane, and stromal amniotic membrane. Differences in cell growth and differentiation on these membranes were investigated. The other aim was to determine whether these membranes enabled urothelial formation. The most rapid cell proliferation and best differentiation were detected on the stromal amniotic membrane. This type of the human amniotic membrane scaffold also supported the formation of engineered urothelium.30
Clinical results
Four studies described the application of engineered tissues in humans for urethral reconstruction (Table 3).
Table 3.
Overview of studies describing clinical trials in the context of urethral TE
| References | No. of patients | Cell type | Scaffolds | Urethral pathology | Follow-up (months) | Results |
|---|---|---|---|---|---|---|
| Fossum et al.42 | 6 | Autologous urothelial cells (bladder washings) | Acellular dermis | Boys with scrotal or perineal hypospadias | 72–103 | Successful in 5/6 patients at first attempt. Additional urethrotomy needed in 1/6 patients. Cosmetical appearance satisfactory in 6/6 patients. |
| Osman et al.43 | 5 | Autologous oral fibroblasts and keratinocytes | Cadaveric de-epidermised dermis | complex strictures | 110–115 | Successful in 3/5 patients, unsuccesful in 1/5 patients, unknown result in 1/5 patients. |
TE: tissue engineering.
Cadaveric de-epidermized dermis was used as a scaffold in a study carried out by Osman et al.43 Autologous oral fibroblasts and keratinocytes were seeded on the scaffold and used for the treatment of complex urethral strictures. Five patients underwent tissue-engineered buccal mucosa urethroplasty in 2004 and were subsequently followed-up on a year basis for approximately nine years. Out of the five patients, grafts remained in situ in four patients during nine-year follow-up, one needed the excision due to entire graft scarring. Another patient underwent partial excision due to hyperproliferation of the graft. Intervention for renarrowing and intermittent self-calibration was needed in three patients. All in all, satisfactory results were found in three of five patients, although further interventions were also needed.
Another study described the use of autologous urothelial cells seeded on acellular dermis to engineer autologous transplant. Cells were obtained from the bladder biopsy and engineering of the transplant was performed three weeks prior to the urethral reconstruction. Six boys born with severe scrotal or perineal hypospadias and pronounced chordee underwent the surgical treatment. The first procedure was the repair of the chordee and urethroplasty was performed seven to fifteen months later. The success of the outcome was evaluated due to cosmetic appearance, voiding function, urinary flow, artificial erection, urethroscopy, and biopsies. Analyses showed that cultured urothelial cells faced the lumen of neourethra. First attempt success of integrated neourethra was found in five patients. One boy needed additional urethrotomy. Patients were followed up six to eight years.42
Discussion
This review article summarizes recent articles in urethral TE. As can be seen, various TE approaches are used in order to replace missing or damaged part of the urethra. The use of buccal mucosa graft is feasible, the surgical technique is established, but has certain limitations – harvesting of the buccal graft is painful, with the risk of future complications (scarring, bleeding, infection, etc.). To counteract these limitations, TE provides several possibilities in damaged urethral repair for the clinical use, including the use of scaffolds, and cell-seeded scaffolds. Cell culture techniques, using adipose-derived stem cells, urine-derived stem cells, human urothelial cells, human umbilical cord mesenchymal stem cells, and keratinocytes were studied. To reduce invasivity, autologous urinary stem cells and autologous adipose-derived stem cells show much promise, as they can be expanded in vitro. Studies above show that proliferation and differentiation of cultured cells are affected by both the culture conditions and type of scaffold. These two factors – culture conditions and type of scaffold used go along with another crucial factor in a clinical setting – vascularization of engineered graft which remains a challenge. After choosing the cell source and cell culture technique, the choice of a scaffold (biological, synthetic, composite) is another determinant of the clinical outcome. Scaffolds that were currently used (PLCL, collagen-based tubes and matrices, electrospun silk fibroin matrices, amniotic membrane, composite scaffolds-PCL/silk fibroin/collagen electrospun nanofiber scaffold, poly(L-lactide)/poly(ethylene glycol), other scaffolds – nanostructured titanium dioxide (TiO2) scaffolds, Wnt pathway inhibitor delivering collagen/poly(L-lactide-co-caprolactone) show various results. Biological scaffolds imitate structure and extracellular molecules of a native scaffold, but their origin, as well as the decellularization process, limits their use in the clinical setting. Yet, there is no standardized or optimal scaffold that could be recommended for clinical trials. 3D bioprinting, in which extracellular matrix is printed together with cell-containing hydrogels,44 and 3D bioprinting of tissue constructs using bio ink45 may overcome these limitations. Moreover, it will be possible to incorporate more types of cells into the artificial tissue of urethra. For example, epithelial cells which secrete various basement membrane components and so support its restoration.
Non-seeded acellular grafts for the urethral replacement show poor or conflicting clinical results.46,47 Cell-seeded biological, composite, or synthetic scaffolds show promising results in various animal models or pediatric patients.40,42,43
In the near future, the further development of urethral TE will be associated mainly with introducing new composite biomaterial and 3D bioprinting technology. Moreover, the great development can be expected in construction of new bioreactors providing dynamic cultivation conditions which may affect the cell proliferation and differentiation. Their combination will lead into design of artificial urethras with strictly defined architecture similar to patient’s native urethra which should be utilized in human medicine.
This review has limitations. The literature search was focused on TE of the urethral tissue in order to limit other studies that can, on the other hand, add some information to urethral TE. The search was limited to the last five years, but some interesting studies were conducted previously. Our aim was to get the most updated information in urethral TE, to summarize the recent development of the urethral TE.
Conclusion
Recent development in the urethral tissue engineering provides a rationale for further development and experiments in a clinical setting. Experience with differentiation of stem cells is growing. Cell-seeded grafts have encouraging clinical results, but optimal protocol-based cell expansion and optimal scaffold for urethral replacement have to be established. 3D bioprinting with cell-laden tissue constructs shows much promise. However, more clinical experience is warranted to be fully established as a therapeutical option in a clinical setting.
Authors’ contributions
SZ and LD participated in initial discussions about the systems and development of concepts presented herein. LD and MG performed database screening and selected articles included in present review. All authors were involved in writing the manuscript. All authors reviewed, edited, and approved of the final submission.
Funding
The present work was supported by the grant APVV-15-0111.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Warwick RT. Urethral surgery. In: Mundy AR. (ed). Current operative surgery: urology, London: Balliere Tindall, 1988, pp. 160–218. [Google Scholar]
- 2.Mangera A, Chapple C. Management of anterior urethral stricture: an evidence-based approach. Curr Opin Urol 2010; 20: 453–8. [DOI] [PubMed] [Google Scholar]
- 3.Davis NF, Quinlan MR, Bhatt NR, Browne C, MacCraith E, Manecksha R, Walsh MT, Thornhill JA, Mulvin D. Incidence, cost, complications and clinical outcomes of iatrogenic urethral catheterization injuries: a prospective multi-institutional study. J Urol 2016; 196: 1473–7. [DOI] [PubMed] [Google Scholar]
- 4.Barbagli G, Lazzeri M. Surgical treatment of anterior urethral stricture diseases: brief overview. Int Braz J Urol 2007; 33: 461–9. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay S, Ringuette-Goulet C, Langlois A, Bolduc S. Clinical challenges in tissue-engineered urethral reconstruction. Transl Androl Urol 2016; 5: 267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steenkamp JW, Heyns CF, de Kock ML. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J Urol 1997; 57: 98–101. [PubMed] [Google Scholar]
- 7.Dubey D. The current role of direct vision internal urethrotomy and self-catheterization for anterior urethral strictures. Ind J Urol 2011; 27: 392–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wein AJ, Kavoussi LR, Partin AW, Peters CA. Campbell-Walsh urology, 11th ed Philadelphia: Elsevier, 2015, pp. 4168–4168. [Google Scholar]
- 9.Dubey D, Vijjan V, Kapoor R, Srivastava A, Mandhani A, Kumar A, Ansari MS. Dorsal onlay buccal mucosa versus penile skin flap urethroplasty for anterior urethral strictures: results from a randomized prospective trial. J Urol 2007; 178: 2466–9. [DOI] [PubMed] [Google Scholar]
- 10.Santucci R, Zimmerman WB. Buccal mucosa urethroplasty for adult urethral strictures. Ind J Urol 2011; 27: 364–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha RJ, Singh V, Sankhwar S, Dalela D. Donor site morbidity in oral mucosa graft urethroplasty: implications of tobacco consumption. BMC Urol 2009; 9: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Kemp V, de Graaf P, Fledderus JO, Ruud Bosch JLH, de Kort LMO. Tissue engineering for human urethral reconstruction: systematic review of recent literature. PLoS One 2015; 10: e0118653–e0118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuai W, Zhongliang C, Guohua L, Xinfeng Z, Liang Z, Yingjian Z, Jiang Z. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Analyt Cell Pathol 2013; 36: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Xu YM, Liu ZS, Li HB. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology 2013; 81: 1075–80. [DOI] [PubMed] [Google Scholar]
- 15.Lang R, Liu G, Shi Y, Bharadwaj S, Leng X, Zhou X, Liu H, Atala A, Zhang Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS One 2013; 8: e53980–e53980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun D, Yang Y, Wei Z, Xu Y, Zhang X, Hong B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J Cell Mol Med 2014; 18: 434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun SY, Kim BS, Kwon SY, Park SI, Song PH, Yoo ES, Kim BW, Kwon TG, Kim HT. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J Korean Med Sci 2015; 30: 301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Fu Q, Zhao RY, Deng CL. Muscular tubes of urethra engineered from adipose-derived stem cells and polyglycolic acid mesh in a bioreactor. Biotechnol Lett 2014; 36: 1909–16. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Xu M, Zhou Z, Zhang K, Zhou J, Zhao Y, Wang Z, Lu M. The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation. PLoS One 2014; 9: e95583–e95583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Xu Y, Xie H, Li C, Song L, Feng C, Zhang Q, Xie M, Wang Y, Lv X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: an animal model. Tissue Eng Part A 2014; 20: 774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang HS, Choi SH, Kim BS, Choi JY, Park GB, Kwon TG, Chun SY. Advanced properties of urine derived stem cells compared to adipose tissue derived stem cells in terms of cell proliferation, immune modulation and multi differentiation. J Korean Med Sci 2015; 30: 1764–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogovaya OS, Fayzulin AK, Vasiliev AV, Kononov AV, Terskikh VV. Reconstruction of rabbit urethral epithelium with skin keratinocytes. Acta Nat 2015; 7: 70–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Sartoneva R, Haaparanta AM, Lahdes Vasama T, Mannerstrom B, Kellomaki M, Salomaki M, Sandor G, Seppanen R, Miettinen S, Haimi S. Characterizing and optimizing poly-L-lactide-co-caprolactone membranes for urothelial tissue engineering. J Royal Soc Interf 2012; 9: 3444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micol LA, Arenas da Silva LF, Geutjes PJ, Oosterwijk E, Hubbell JA, Feitz WFJ, Frey P. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012; 33: 7447–55. [DOI] [PubMed] [Google Scholar]
- 25.Sayeg K, Freitas-Filho LG, Waitzberg AFL, Arias VEA, Laks M, Egydio FM, Oliveira AS. Integration of collagen matrices into the urethra when implanted as onlay graft. Int Braz J Urol 2013; 39: 414–23. [DOI] [PubMed] [Google Scholar]
- 26.Orabi H, AbouShwareb T, Zhang Y, Yoo JJ, Atala A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol 2013; 63: 531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Cano F, Garzón I, Marañés C, Liceras E, Martín-Piedra MA, Ruiz-Montes AM, Alaminos M, Fernández-Valadés R. Histological and immunohistochemical changes in the rat oral mucosa used as an autologous urethral graft. J Pediat Surg 2016; 48: 1557–64. [DOI] [PubMed] [Google Scholar]
- 28.Xie M, Song L, Wang J, Fan S, Zhang Y, Xu Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J Surg Res 2013; 184: 774–81. [DOI] [PubMed] [Google Scholar]
- 29.Chung YG, Tu D, Franck D, Gil ES, Algarrahi K, Adam RM, Kaplan DL, Estrada CR, Mauney JR. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One 2014; 9: e91592–e91592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerman UD, Veranič P, Kreft ME. Amniotic membrane scaffolds enable the development of tissue-engineered urothelium with molecular and ultrastructural properties comparable to that of native urothelium. Tissue Eng Part C 2013; 20: 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Liu T, Yang L, Zhang G, Liu H, Yi X, Yang X, Lin TY, Qin W, Yuan J. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med Sci Monit 2014; 20: 2430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei G, Li C, Fu Q, Xu Y, Li H. Preparation of PCL/silk fibroin/collagen electrospun fiber for urethral reconstruction. Int Urol Nephrol 2015; 47: 95–9. [DOI] [PubMed] [Google Scholar]
- 33.Kajbafzadeh AM, Sabetkish S, Tourchi A, Amirizadeh N, Afshar K, Abolghasemi H, Elmi A, Talab SS, Eshghi P, Mohseni MJ. The application of tissue-engineered preputial matrix and fibrin sealant for urethral reconstruction in rabbit model. Int Urol Nephrol 2014; 46: 1573–80. [DOI] [PubMed] [Google Scholar]
- 34.Heller M, Frerick-Ochs EV, Bauer HK, Schiegnitz E, Flesch D, Brieger J, Stein R, Al-Nawas B, Brochhausen C, Thüroff JW, Unger RE, Brenner W. Tissue engineered pre-vascularized buccal mucosa equivalents utilizing a primary triculture of epithelial cells, endothelial cells and fibroblasts. Biomaterials 2016; 77: 207–15. [DOI] [PubMed] [Google Scholar]
- 35.Imani R, Pazoki M, Zupančič D, Kreft ME, Kralj-Iglič V, Veranič P, Iglič A. Biocompatibility of different nanostructured TiO2 scaffolds and their potential for urologic applications. Protoplasma 2016; 253: 1439–47. [DOI] [PubMed] [Google Scholar]
- 36.Jia W, Tang H, Wu J, Hou X, Chen B, Chen W, Zhao Y, Shi C, Zhou F, Yi W, Huang S, Ye G, Dai J. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015; 69: 45–55. [DOI] [PubMed] [Google Scholar]
- 37.Zhang K, Guo X, Zhao W, Niu G, Mo X, Fu Q. Application of Wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: in vitro and in vivo study. Int J Mol Sci 2015; 16: 27659–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv X, Guo Q, Han F, Chen C, Ling C, Chen W, Li B. Electrospun poly(l-lactide)/poly(ethylene glycol) scaffolds seeded with human amniotic mesenchymal stem cells for urethral epithelium repair. Int J Mol Sci 2016; 17: 1262–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv X, Li Z, Chen S, Xie M, Huang J, Peng X, Yang R, Wang H, Xu Y, Feng C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016; 84: 99–110. [DOI] [PubMed] [Google Scholar]
- 40.Aufderklamm S, Vaegler M, Kelp A, Maurer S, Gustafsson L, Mundhenk J, Busch S, Daum L, Stenzl A, Amend B, Sievert KD. Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: first results in a xenograft minipig model. World J Urol 2017; 35: 1125–32. [DOI] [PubMed] [Google Scholar]
- 41.Pinnagoda K, Larsson HM, Vythilingam G, Vardar E, Engelhardt EM, Thambidorai RC, Hubbell JA, Frey P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater 2016; 43: 208–17. [DOI] [PubMed] [Google Scholar]
- 42.Fossum M, Skikuniene J, Orrego A, Nordenskjöld A. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells: hypospadias repair with cultured cells. Acta Paediat 2012; 101: 755–60. [DOI] [PubMed] [Google Scholar]
- 43.Osman NI, Patterson JM, MacNeil S, Chapple CR. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur Urol 2014; 66: 790–1. [DOI] [PubMed] [Google Scholar]
- 44.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 2014; 26: 3124–30. [DOI] [PubMed] [Google Scholar]
- 45.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M, Khademhosseini A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater 2016; 28: 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hauser S, Bastian PJ, Fechner G, Müller SC. Small intestine submucosa in urethral stricture repair in a consecutive series. Urology 2006; 68: 263–6. [DOI] [PubMed] [Google Scholar]
- 47.le Roux PJ. Endoscopic urethroplasty with unseeded small intestinal submucosa collagen matrix grafts: a pilot study. J Urol 2005; 173: 140–3. [DOI] [PubMed] [Google Scholar]