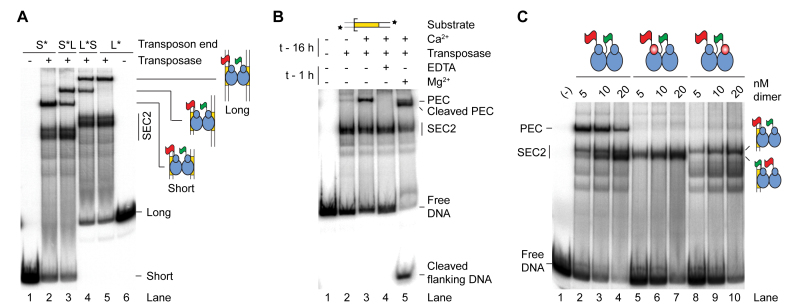
Figure 3.
Single-chain dimer and the paired ends complex. (A) Identification of the PEC using the transposon end ‘long-by-short’ strategy. Complexes were formed with the single-chain dimer in the presence of combinations of long (L) and short (S) transposon ends that were either unlabeled or radioactively labeled, as indicated by the asterisk. The presence of two transposon ends in the PEC is revealed by the appearance of a band of intermediate mobility in reactions that contain a mixture of long and short substrates. (B) The single-chain dimer assembles a Ca2+-dependent PEC that is catalytically active. Complexes were assembled overnight in the presence or absence of Ca2+. Where indicated, EDTA or the catalytic metal ion Mg2+ was added for 1 h before the complexes were separated by electrophoresis. (C) The PEC most likely contains a single transposase dimer. Complexes were assembled with the wild-type single-chain dimer or mutant single-chain dimers that carried a mutation in the DNA-binding domain (R104A) of one of the transposase subunits (red ovals). The two structural isoforms of SEC2, in which the first (MBP-tagged) or the second (TrxA-tagged) subunit of the dimer is bound in cis to the transposon end, are indicated. The decreasing amount of PEC at the expense of SEC2 with increasing transposase concentration is a predicted feature of our model of mariner autoregulation (28) (see Figure 6A). For a characterization of the single-chain dimer see also Supplementary Figure S1.