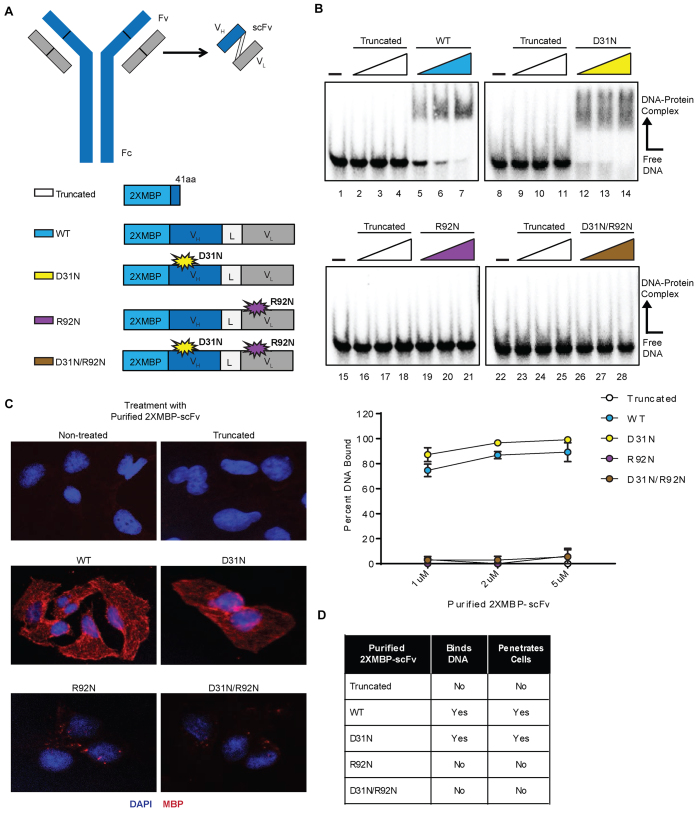
Figure 1.
Purification and biochemical characterization of 3E10-scFv proteins supports the link between 3E10’s DNA binding ability and capacity for cellular penetration. (A) Schematic of the 3E10 full length antibody as well as the derived scFv. The heavy chain (VH) and light chain (VL) are connected by a flexible linker (L) to produce the scFv. The scFv was cloned into a 2XMBP_phCMV1 expression vector. Various point mutations were made to produce five total 2XMBP-scFv proteins. Expression constructs for each scFv and their respective point mutations are schematized. (B) An electrophoretic mobility shift assay was performed using radiolabeled ssDNA to determine the DNA binding ability of all five purified proteins. The assay was performed using a titration of 1, 2 and 5 μM of each purified scFv. Representative images and quantification are shown. (C) U2OS cells were treated with the five scFv proteins and the cellular penetration ability of each was observed via immunofluorescence. (D) A summary of the biochemical properties of each of the five purified scFvs is provided.