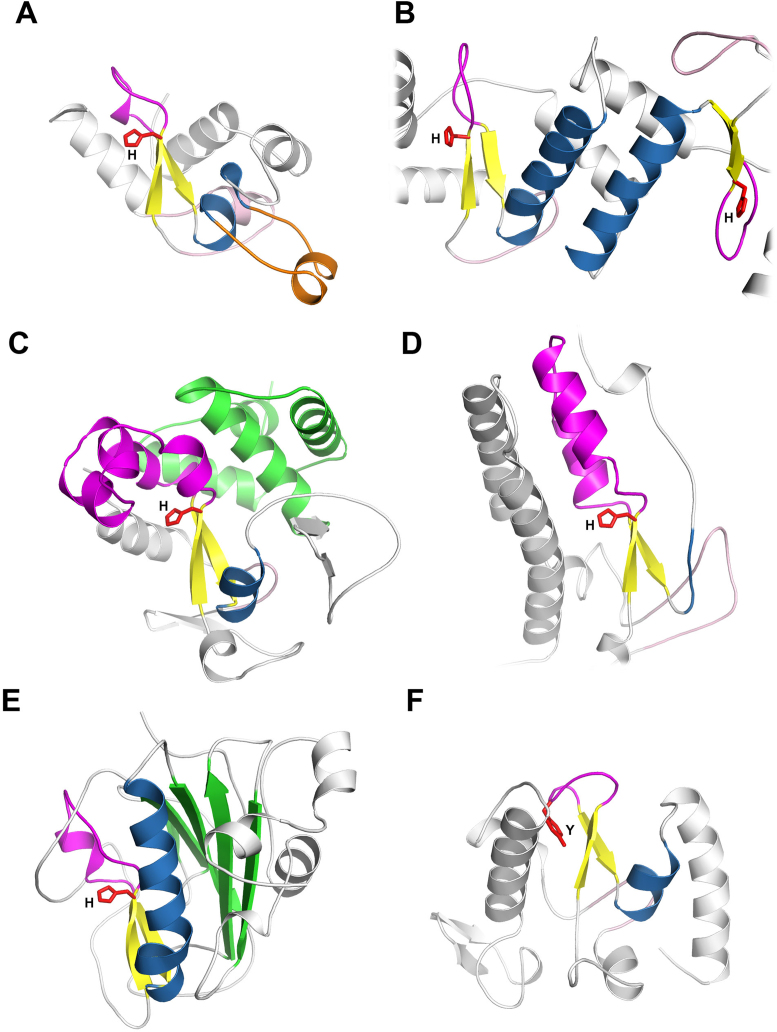
Figure 3.
Structural diversity of His-Me fingers. The core elements of the fold (ββα motif) are coloured in yellow and blue for β-strands and α-helix, respectively. The Ω-loop is coloured in magenta. The zinc knuckle preceding the core β-hairpin is shown in light pink. The catalytic site His/Tyr is presented as red sticks. (A) The structure of viral RecA-dependent Ref nuclease (PDB ID: 3plw) with finger loop insertion (orange). (B) Dimerized nuclease domains of Holliday Junction Resolvase ENDOVII (PDB ID: 2qnc). (C) The structure of Vibrio vulnificus nuclease (Vvn) (PDB ID: 1oup) with the α-helical domain (green) at the back of the HNH domain. (D) The structure of Caspase-Activated DNase (CAD) (PDB ID: 1v0d). The loop substituting for the core α-helix is coloured in blue. (E) The structure of Serratia marcescens nuclease (Sm) (PDB ID: 1g8t) with the β-sheet domain (green) at the back of HNH domain. (F) The structure of PacI (PDB ID: 3m7k) with tyrosine substituting for catalytic histidine.