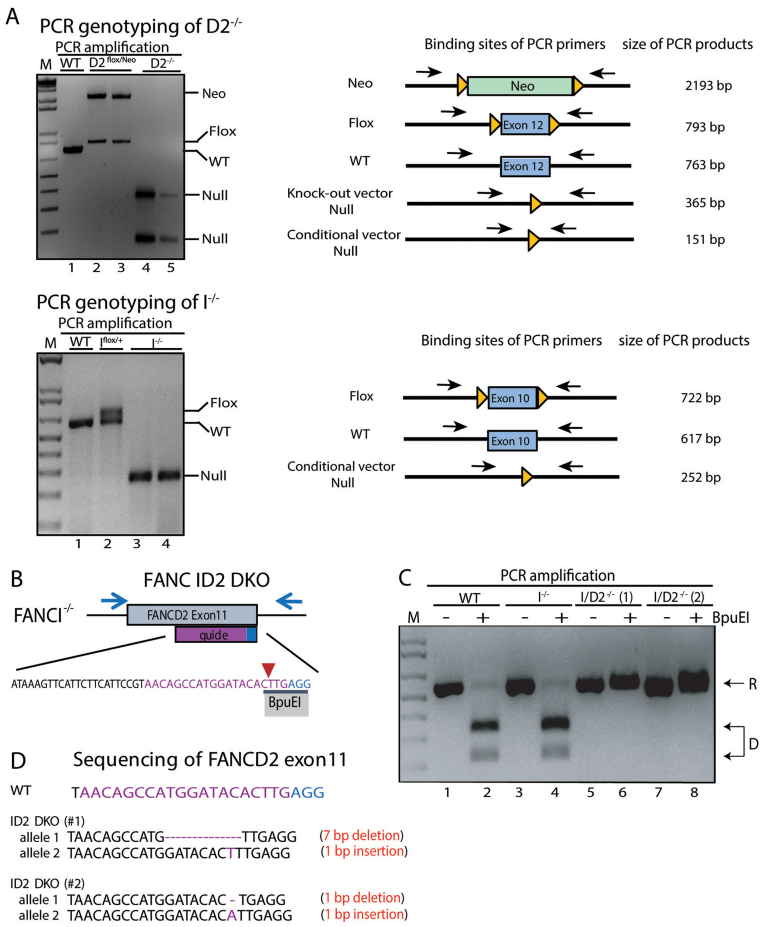
Figure 2.
Confirmation of D2−/−, I−/− and ID2 DKO cell lines. (A) PCR genotyping of D2−/− and I−/− cells and targeting intermediates. Left panel: analyses of DNA fragments for WT (lane 1), D2flox/Neo (lanes 2 and 3) and D2−/− (lanes 4 and 5) after PCR amplification with primers FANCD2_EX11SF and FANCD2_LoxPSR flanking the targeted exon. Right panel: analyses of DNA fragments after PCR amplification from WT (lane 1), Iflox/+ (lane 2) and I−/− (lanes 3 and 4) cells using primers FancIc_GG_LIF and FancIcond_GG_LoxR flanking the targeted exon. The PCR amplification spanning the targeted exons (exon 12 in FANCD2; exon 10 in FANCI) was used to confirm the removal of the respective exon in the D2−/− and I−/− cell lines. M: 1 kb markers. (B) Schematic of the FANCD2 targeting strategy in I−/− cells. CRISPR/Cas9-mediated gene targeting was used to functionally inactivate FANCD2 in the I−/− cell line. A guide RNA (purple sequence) was designed targeting FANCD2 exon 11 with the Cas9 cut site (red arrow) overlapping an endogenous BpuEI restriction enzyme recognition site (black bar). The PAM sequence of the sgRNA is shown in blue. Indels introduced at the Cas9 cut site should disrupt the BpuEI cleavage site. (C) Genotyping of ID2 DKO cells. PCR amplification and BpuEI restriction enzyme digestion of FANCD2 exon 11 in WT, I−/− and two ID2−/− clones (1 and 2). Analyses of DNA fragments after PCR amplification with primers FancD2_CC_ F2 and FancD2_CC_ R2 (blue arrows from panel B) from WT (lanes 1 and 2), I−/− (lanes 3 and 4) and ID2 DKO cells (lanes 5, 6, 7 and 8) that had been untreated (lanes 1, 3, 5 and 7) or treated with BpuEI (lanes 2, 4, 6 and 8). Cleavage by BpuEI produces two faster migrating fragments (D, lanes 2 and 4). Resistance (R) to BpuEI digestion is seen in lanes 6 and 8 with the two ID2 DKO clones. (D) Sequence confirmation of CRISPR/Cas9 induced bi-allelic frameshift mutations in FANCD2 in the two ID2 DKO clones #1 and #2.