Abstract
Developing a better understanding of how and under what circumstances alcohol affects the emotions, cognitions and neural functions that precede and contribute to dangerous behaviors during intoxication may help to reduce their occurrence. Alcohol intoxication has recently been shown to reduce defensive reactivity and anxiety more during uncertain vs certain threat. However, alcohol’s effects on emotionally motivated attention to these threats are unknown. Alcohol may disrupt both affective response to and attentional processing of uncertain threats making intoxicated individuals less able to avoid dangerous and costly behaviors. To test this possibility, we examined the effects of a broad range of blood alcohol concentrations on 96 participants’ sub-cortically mediated defensive reactivity (startle potentiation), retrospective subjective anxiety (self-report) and cortically assessed emotionally motivated attention (probe P3 event related potential) while they experienced visually cued uncertain and certain location electric shock threat. As predicted, alcohol decreased defensive reactivity and subjective anxiety more during uncertain vs certain threat. In a novel finding, alcohol dampened emotionally motivated attention during uncertain but not certain threat. This effect appeared independent of alcohol’s effects on defensive reactivity and subjective anxiety. These results suggest that alcohol intoxication dampens processing of uncertain threats while leaving processing of certain threats intact.
Keywords: attention, uncertainty, stress, alcohol, threat, anxiety
Introduction
According to recent calculations, problematic alcohol use cost the United States 249 billion in 2010 alone, a cost that continues to increase (Sacks et al., 2015). Dangerous and damaging behavior by a subset of intoxicated individuals makes up a large portion of this cost (e.g. medical bills from preventable injury, drunk driving, petty crime). Before we can change these behaviors, we may require better understanding of how alcohol affects the cognitive and affective processes that precede and influence them. Research into how and under what circumstances alcohol affects our emotions, attention and their neural substrates will help inform prevention and intervention efforts to decrease the risk and cost of intoxicated behavior.
An intoxicated individual may, for example, decide to walk home alone on poorly lit or otherwise unsafe streets, pursue a risky sexual encounter, or commit petty theft (e.g. shoplift)–all actions he or she might normally avoid while sober. The threats of assault or robbery, contracting a sexually transmitted disease, or legal consequences may generally motivate sober individuals to refrain from risky behaviors. However, these same threats appear to have less influence on intoxicated individuals for reasons that are still inadequately understood. Notably, these threats all share some degree of uncertainty. Street robberies or assaults are infrequent, symptoms of sexually transmitted diseases are often invisible, and petty theft is often not detected or punished legally. In fact, many threats in our daily life are characterized by some degree of uncertainty. In light of this, how people respond to uncertain threats in particular has recently become an important focus of cognitive, affective and neuroscience research (Davis et al., 2010; Grupe and Nitschke, 2013).
Laboratory research on the neurobehavioral, cognitive and affective responses to threats distinguishes between those that are uncertain vs certain. These two types of threats elicit distinct patterns of innate defensive behaviors that involve activation of overlapping but separable sub-nuclei within the central extended amygdala (Davis, et al., 2010). Through these varying pathways in the central amygdala, the eye blink startle response to auditory ‘startle probes’ is potentiated during presentation of visual cues signaling threat of both uncertain and certain shock (Davis, 2006). However, startle potentiation is selectively decreased during threat of uncertain shock by anxiolytic drugs such as benzodiazepines and alcohol (Grillon et al., 2006; Kaye et al., 2017). In fact, across several manipulations of threat certainty, alcohol has consistently produced a significantly greater reduction of startle potentiation when the threats were in some way uncertain, regardless of what was actually uncertain about the threats (Kaye et al., 2017). Uncertain threats also elicit subjective emotional (e.g. anxious) responses (Jackson et al., 2015), and alcohol, an anxiolytic drug, reduced self-reported anxious response more during uncertain than certain threats in one study (Bradford et al., 2013). Furthermore, recent data suggests that alcohol is more likely to reduce negative affect in social settings when the behavior of others is uncertain (e.g. social attribution theory, Fairbairn and Sayette, 2014).
Uncertain and certain threats each may also affect attentional processes differently (Cornwell et al., 2008; Blanchard et al., 2011; but see Nelson et al., 2015). Alcohol has been observed to impair attention in some studies and not others (see Sayette, 2017 for review), but the specific circumstances under which alcohol has these detrimental effects remains unclear (e.g. Farris et al, 2008). No study to our knowledge has assessed alcohol’s effects on attentional processing of uncertain vs certain threats.
For the sober individual, threats or other emotionally relevant stimuli demand attention, a process thought to be adaptive for survival (Blanchard et al., 2011; Robinson et al., 2015). Researchers can unobtrusively measure this attentional demand using Event Related Potentials (ERPs). The obligatory P3 is a classic ERP usually elicited in response to unexpected or infrequent stimuli and is believed to reflect, among other things, attentional processing (see Linden, 2005 for review). However, when participants’ attentional resources are engaged by an emotionally relevant foreground stimulus or context (e.g. presentation of a frightening picture or a cue that signals impending shock), the P3 to background, task-irrelevant stimuli (e.g. an occasional innocuous auditory tone) is suppressed (Schupp et al., 1997; Keil et al., 2007). Given putative limits on attentional resources, more attentionally engaging foreground emotional stimuli or contexts result in greater suppression of the P3 to background stimuli (Hamm et al., 2007; Ferrari et al., 2010).
Researchers have recently taken advantage of this suppression of the P3 as a ‘relatively pure index’ of emotionally motivated attention that is conveniently assessed in paradigms using startle potentiation that already include infrequent, task-irrelevant background stimuli (i.e. the auditory startle probes; Bradley et al., 2006). In prior research, the P3 ERP elicited from the task irrelevant, auditory startle probes was more suppressed in sober individuals when they viewed threatening or otherwise emotionally evocative stimuli compared to when they viewed neutral stimuli (i.e. probe P3 suppression; Schupp et al., 1997; Nelson et al., 2015). Using these methods, researchers have recently shown increased allocation of emotionally motivated attention during presentation of visual cues signaling both uncertain and certain shock relative to during presentation of no-shock cues (Nelson et al., 2015). This increased allocation of attentional resources during presentation of cues that signal both uncertain and certain threats over presentation of more benign stimuli may aid sober individuals to fully process threats and select adaptive responses in threatening situations.
Emotionally motivated attention and associated elaborative processing of uncertain threats may also be affected by alcohol. Alcohol may disrupt both affective response and attentional processing to uncertain threats making intoxicated individuals less able to avoid dangerous and costly behaviors in these situations. Because affective and attentional processes that influence behavior may not always reach conscious awareness, unobtrusive, nonconscious measure of these processes in a paradigm that allows parametric manipulation of threat certainty may be a valuable tool for understanding these processes.
Here, we report the first assessment of alcohol’s effects on emotionally motivated attention during cued threat of uncertain and certain electric shocks using probe P3 suppression. We simultaneously measured startle potentiation and self-reported anxiety during these threat cues to evaluate relationships between alcohol’s effects on attentional processing, defensive reactivity and subjective emotional response. This also allowed us to assess potential unique effects of alcohol on attentional processing, controlling for defensive reactivity and subjective emotional response. We assessed alcohol’s effects across a broad range of blood alcohol contents (BACs) to evaluate dose response and better model real-world drinking levels (Bradford et al., 2013). Based on prior research (e.g. Bradford et al., 2013), we predicted alcohol would produce a greater dose-dependent reduction of startle potentiation and self-reported anxiety during uncertain compared to certain location threats. We did not offer a priori predictions about alcohol’s effect on attentional processing but instead tested for this effect, both overall and when controlling for alcohol’s influence on startle potentiation and self-reported anxiety.
Method
Open science
Following recommendations about research transparency (Simmons et al., 2012), we have reported how we determined our sample size, all data exclusions, all manipulations and all measures in the study. Following emerging open science guidelines (Schönbrodt et al., 2015), we have made the data and analysis scripts, associated with this report publicly available via Open Science Framework at osf.io/5n7hm.
Participants
The study was approved by the Institutional Review Board’s Social and Behavioral Sciences human subjects committee at the University of Wisconsin, and informed written consent was obtained from all study participants prior to their participation. We recruited 96 participants (48 female; mean age = 22.5 years, s.d. = 2.4 years) from the university community. We discarded and replaced data from an additional 6 participants before analysis due to data collection and/or equipment failure. Power analyses indicated that a sample size of 87 participants would provide 80% power to detect a medium effect size (partial eta-squared = 0.09) for the BAC X Threat type interactions for our three dependent measures, which is comparable to effect sizes seen in recent studies using similar tasks (e.g. Bradford et al., 2013). We planned to recruit 96 participants to balance cell sizes across target doses, task orders, and gender. Participants were between 21 and 35 years old, had experience within the last year with drinking the amount of drinks needed to obtain the highest study dose of alcohol, reported no history of alcohol-related problems, no current psychiatric medication use, no alcohol contraindicated medical condition, were not pregnant (verified by urine sample) and were sober on arrival (verified via breathalyzer [Alcosensor IV; Intoximeters Inc., St. Louis, MO)]. We paid participants $10/h or class extra-credit points for their participation.
Baseline general startle reactivity assessment
We presented visual and auditory stimuli with a PC-based Matlab script using the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). Prior to beverage assignment, we measured participants’ general startle reactivity in a shock-free baseline procedure lasting 4 min (Bradford et al., 2014a; see Startle Response Measurement and Processing below). Participants viewed a series of 12 gray scale squares with no instructed meaning presented in the center of a CRT monitor for 5 s each separated by an inter-trial interval (ITI, range 10–20 s, M = 15 s).
Beverage manipulation
In a between-subjects manipulation, we randomly administered 4 alcohol doses (target BACs of 0.03, 0.06, 0.09 and 0.12; N = 16 each) and a placebo dose (N = 32) across the 96 participants. Alcohol doses were equally stratified by sex. Beverage manipulation methods were consistent with previous research (Bradford et al., 2013; see Supplementary Materials for more information). In analyses evaluating alcohol’s effects on dependent variables, we used mean achieved BAC averaged across BAC measurements for each participant taken with the breathalyzer immediately before start and after completion of the main task.
Shock procedures
After the beverage manipulation, we measured participants’ subjective tolerance to a series of increasing intensity 200 ms electric shocks (7 mA maximum) using a custom shock box following standard procedures from our laboratory (Bradford et al., 2014b; see Supplementary Materials for more information). We used participants’ maximum tolerated shock intensity at each location (i.e. the right triceps and calf) for shocks in the main task to minimize potential effects of individual differences in subjective shock tolerance.
Cued threat of shock task
In the main task, participants viewed a series of serially presented gray-scale square visual cues presented on a CRT monitor for 5 s each and separated by an ITI (range 10–20 s, M = 15 s). Visual cues were presented in within-subject blocks with the same visual cue repeated three times within each block. Blocks consisted of cues that signaled impending shocks to a certain bodily location, shocks to an uncertain bodily location, or cues that signaled no-shock at all. In the center of the gray-scale square, a two-character abbreviation indicated which body location shocks would occur on (see below).
Each participant viewed four different certain location shock blocks consisting of cues signaling which of four specific bodily locations would receive an impending electric shock: the participant’s right triceps (indicated with the characters ‘RT’), left triceps (‘LT’), right calf (‘RC’) and left calf (‘LC’). We instructed participants that electric shocks at the indicated location would be administered at the end of the 5 s cues for all certain-location shock blocks.
Participants also viewed four uncertain location shock blocks in which all the cues included the characters ‘??’. We instructed participants that the location of shock would vary across these uncertain location cues within each uncertain block. In fact, all four shock locations (right triceps, left triceps, right calf and left calf) in the uncertain location blocks were equiprobable and intermixed pseudo-randomly with none of the shock locations repeated more than once within a block. In all shock blocks, 200 ms shocks were administered at 4.8 s post-cue onset.
Finally, participants viewed four no-shock blocks (including the characters ‘NS’). We instructed participants that no shocks would be administered in no-shock blocks and during ITIs in any block. A pre-block message on the monitor indicated the start of each block type. Blocks were presented in one of eight pseudo-random orders with half starting with an uncertain block and half starting with each of the four certain blocks, counterbalanced across participants.
After the main task and BAC assessment, participants rated how anxious they were when they saw each cue type, using a 7 point rating scale (1 = ‘not at all anxious’; 7 = ‘extremely anxious’). Participants then completed a placebo manipulation check, a battery of self-report individual difference questionnaires for goals not relevant to the current student (see Supplemental Materials), were debriefed, compensated and dismissed once reaching a BAC below 0.03%.
Startle response measurement and processing
A Neuroscan Synamps bioamplifier (Compumedics Neuroscan, Charlotte, NC) sampled the electromyographic signal at 2500 Hz from two 4 mm Ag-AgCl sensors (TDE-023; Discount Disposables, St. Albans, VT) filled with conductive gel (ECI Electro-Gel; Electro-cap International, Eaton, OH) placed over the orbicularis oculi muscle under the right eye according to published guidelines (Blumenthal et al., 2005; Bradford et al., 2014b).
We measured the eye-blink startle response to acoustic startle probes (50 ms, 102 dB white noise with near instantaneous rise time). We presented 6 noise probes during a subset of the visual cues at 3.5 or 4.5 s post cue onset with equal probable timing during the baseline procedure. We presented 24 noise probes during a subset of the cues in the threat of shock (16 probes; 8 for each threat type) and no-shock (8 probes) blocks at 3.5 or 4.5 s, post cue onset, with equal probable timing during the main task (see Supplementary Materials for more information).
We conducted offline data processing for all physiological signals using the PhysBox plugin (Curtin, 2011) within the EEGLab toolbox (Delorme and Makeig, 2004) in MATLAB (MATLAB and Statistics Toolbox, 2013). We followed published guidelines for startle response reduction and processing (Blumenthal et al., 2005; Bradford et al., 2014b). We excluded from all analyses one participant, whose mean baseline general startle reactivity was <5 microvolts (i.e. non-responder).
Probe P3 measurement and processing
The Neuroscan bioamplifier also sampled the EEG signal at 2500 Hz from nine scalp sites (Fz, F3, F4, Cz, C3, C4, Pz, P3, P4) with conductive gel in a custom Electro-cap (ECI Electro-Gel, Electro-Cap International; Eaton, OH). We referenced signals online to the left mastoid and re-referenced offline to averaged mastoids. We measured vertical electrooculogram (VEOG) activity to correct for eyeblink artifact. We measured the P3 ERP to the acoustic startle probes at electrode site Pz, consistent with most published reports (e.g. Alius et al, 2015; Nelson and Hajcak, 2017). Data from the other electrode sites were collected for goals not relevant to the current study (reported in the Supplementary Materials).
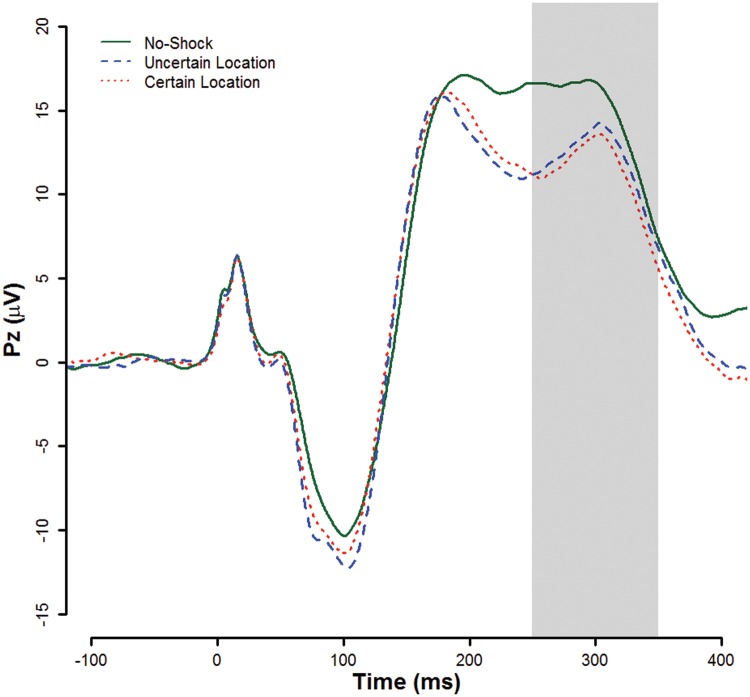
EEG response reduction and processing followed published guidelines (Picton et al., 2000). We removed three participants from ERP analyses due to excessive noise in the VEOG data that prevented use of eyeblink artifact correction. We removed one participant with >25% of trials rejected as EEG artifact from ERP analysis. We selected the scoring window of 250–350 ms post probe to be consistent with previous research (e.g. Benning et al., 2015; see Supplementary Materials for further information). We scored the probe P3 as mean response in this window in each condition (Figure 1).
Fig. 1.
Grand average event related potentials to the auditory startle probe by threat type. Gray band indicates scoring window for probe P3. Figure © 2017 John Curtin, Daniel Bradford, Courtney Motschman, and Mark Starr under Creative Commons Attribution 4.0 International Public License CC-By.
Analysis plan
We calculated general startle reactivity as mean startle response to startle probes during cues in the baseline procedure. We calculated startle potentiation as the increase in startle response to probes during cues in the shock blocks relative to cues in the no-shock blocks, self-reported anxiety as the increase in anxiety to the shock cues relative to the no-shock cues and probe P3 suppression as the decrease in the P3 ERP to probes during cues in the shock blocks relative to cues in the no-shock blocks.
We analyzed startle potentiation, self-reported anxiety, and probe P3 suppression with threat type (mean of response across all uncertain cues–mean of response across all certain cues) as a within-subjects factor in separate, fully interactive, General Linear Models (GLMs) using R Studio (RStudio: Integrated development environment for R, 2016) for R (R Development Core Team, 2015) with the lmSupport (Curtin, 2015) package. All GLMs included quantitative, between-subjects regressors for BAC and general startle reactivity (mean centered). All GLMs also included task block order using unit weighted, centered, orthogonal regressors. We report both partial eta-squared (ηp2) and GLM coefficients (b) to describe effect sizes. We conducted outlier analysis (studentized residual with Bonferroni corrected P < 0.05) in preliminary GLMs for each dependent variable. This resulted in the removal of one outlier each for GLMs involving startle potentiation and self-reported anxiety.
We conducted supplemental analyses to clarify the shape of the alcohol dose response function by adding a regressor to rule out a quadratic BAC effect to each of the GLMs described above (Bradford et al., 2013). We assessed if the BAC X threat type interaction for probe P3 suppression was independent from alcohol’s effects on startle potentiation and self-reported anxiety by testing this interaction controlling for startle potentiation and self-reported anxiety (uncertain–certain) difference scores by including each of these scores additively in separate GLMs otherwise identical to the primary GLM for probe P3. Finally, we tested the bivariate correlations between the uncertain - certain difference scores for all DVs to test for further evidence that each DV contrast reflected distinct processes.
Results
BAC
The mean BAC for participants administered alcohol was 0.048% (s.d. = 0.04%) immediately before the main task, and 0.048% (s.d. = 0.05%) immediately after the task. The placebo manipulation was successful in establishing expectations of alcohol consumption and intoxication across BACs (see Supplementary Materials for full analysis of the placebo manipulation). Observed BAC was multiplied by 100 in the following analysis to increase interpretability of its GLM coefficients, such that a 1-unit increase represented a 0.01% increase in BAC.
Startle potentiation
Spearman-Brown corrected split half (odd vs even trials) internal consistency for startle magnitude was: no-shock rsb = 0.96, certain-threat rsb = 0.97 and uncertain-threat rsb = 0.96 and startle potentiation was: certain-threat rsb = 0.64 and uncertain-threat rsb = 0.65.
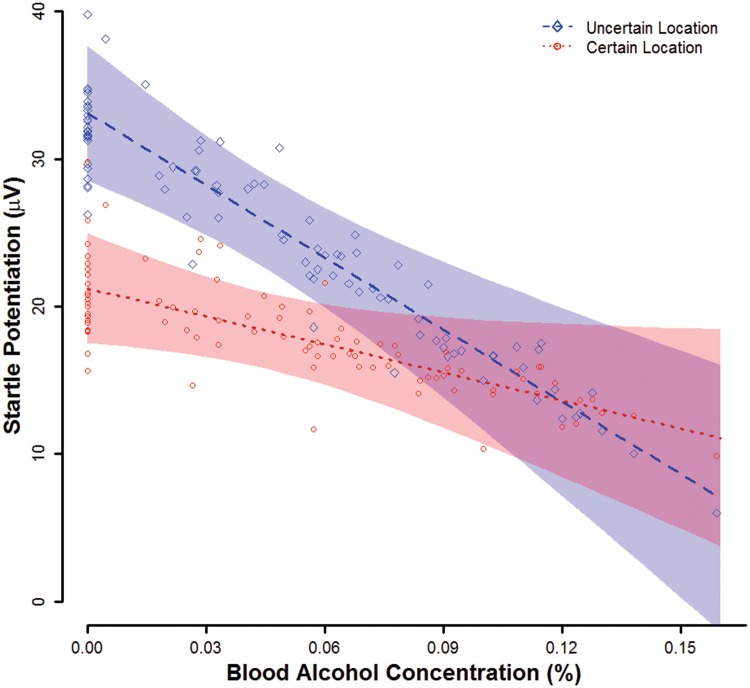
At a BAC of 0.00%, mean startle potentiation was significant (nonzero) overall, ηp2 = 0.36, b = 27.2, t(86) = 6.97, P < 0.001 and separately during uncertain (ηp2 = 0.38, b = 33.1, t(86) = 7.24, P < 0.001) and certain (ηp2 = 0.27, b = 21.2, t(86) = 5.71, P < 0.001) threats (Figure 2). Startle potentiation was significantly greater during uncertain threats than certain threats, ηp2 = 0.16, b = 11.9, t(86) = 4.04, P < 0.001.
Fig. 2.
Startle potentiation by BAC and Threat Type. Lines display point estimates for mean startle potentiation by BAC and threat type from the general linear model. Translucent bands indicate confidence envelopes (±1 SE) for these point estimates. Points represent participants’ startle potentiation residual scores relative to their predicted values and scaled by the square root of N to allow display on the same scale as the population mean point estimates. Figure © 2017 John Curtin, Daniel Bradford, Courtney Motschman, and Mark Starr under Creative Commons Attribution 4.0 International Public License CC-By.
As predicted, the BAC X threat type interaction was significant, ηp2 = 0.05, b = –1.0, t(86) = 2.11, P = 0.038, such that the BAC effect on startle potentiation was significantly greater during uncertain (ηp2 = 0.05, b = –1.6, t(86) = 2.22, P = 0.029) than certain threats (ηp2 = 0.01, b = –0.6, t(86) = 1.06, P = 0.292). The quadratic BAC X threat type effect was not significant, ηp2 = 0.01, b = 0.1, t(85) = 0.86, P = 0.390.
Self-reported anxiety
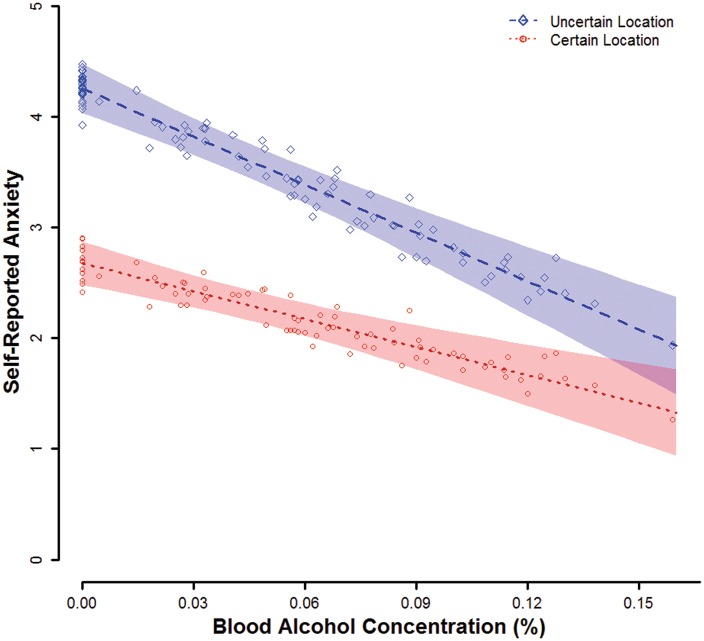
At a BAC of 0.00%, mean retrospective self-reported anxiety was significant (nonzero) overall, ηp2 = 0.78, b = 3.5, t(86) = 17.32, P < 0.001 and separately during uncertain (ηp2 = 0.81, b = 4.3, t(86) = 19.21, P < 0.001) and certain (ηp2 = 0.68, b = 2.7, t(86) = 13.59, P < 0.001) threats (Figure 3). Self-reported anxiety was significantly greater during uncertain threats than certain threats, ηp2 = 0.65, b = 1.6, t(86) = 12.66, P < 0.001.
Fig. 3.
Self-reported anxiety by mean BAC and threat type. Lines display point estimates for mean self-reported anxiety by BAC and threat type from the general linear model. Translucent bands indicate confidence envelopes (±1 SE) for these point estimates. Points represent participants’ self-reported anxiety residual scores relative to their predicted values and scaled by the square root of N to allow display on the same scale as the population mean point estimates. Figure © 2017 John Curtin, Daniel Bradford, Courtney Motschman, and Mark Starr under Creative Commons Attribution 4.0 International Public License CC-By.
As predicted, the BAC X threat type interaction was significant, ηp2 = 0.10, b = –0.1, t(86) = 3.04, P = 0.003, such that the BAC effect on self-reported anxiety was significantly greater during uncertain (ηp2 = 0.16, b = –0.1, t(86) = 4.08, P < 0.001) than certain threats (ηp2 = 0.08, b = –0.1, t(86) = 2.66, P = 0.009). The quadratic BAC X threat type effect was not significant, ηp2 < 0.01, b = 0.0, t(85) = 0.62, P = 0.536.
Probe P3 suppression
Spearman-Brown corrected split half (odd vs even trials) internal consistency for probe P3 magnitude was: no-shock rsb = 0.79, certain-threat rsb = 0.63 and uncertain-threat rsb = 0.73 and probe P3 suppression was: certain-threat rsb = 0.34, and uncertain-threat rsb = 0.47.
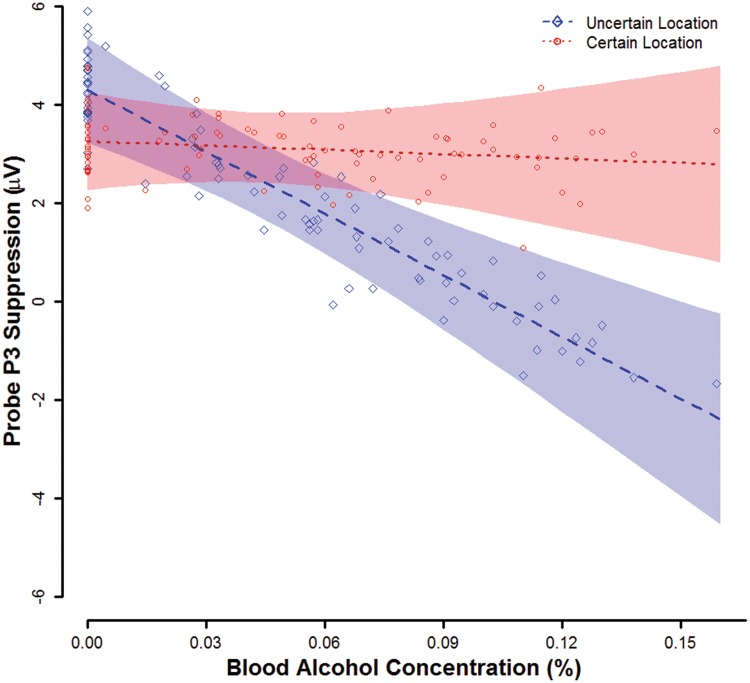
At a BAC of 0.00%, mean startle probe P3 suppression was significant (nonzero) overall, ηp2 = 0.17, b = 3.8, t(83) = 4.06, P < 0.001 and separately for uncertain (ηp2 = 0.16, b = 4.3, t(83) = 4.03, P < 0.001) and certain (ηp2 = 0.11, b = 3.3, t(83) = 3.28, P = 0.002) threats (Figure 4). Probe P3 suppression was comparable during uncertain threats and certain threats, ηp2 = 0.02, b = 1.04, t(83) = 1.17, P = 0.244.
Fig. 4.
Probe P3 Suppression by Mean BAC and Threat Type. Lines display point estimates for mean probe P3 suppression by BAC and threat type from the general linear model. Translucent bands indicate confidence envelopes (±1 SE) for these point estimates. Points represent participants’ probe P3 suppression residual scores relative to their predicted values and scaled by the square root of N to allow display on the same scale as the population mean point estimates. Figure © 2017 John Curtin, Daniel Bradford, Courtney Motschman, and Mark Starr under Creative Commons Attribution 4.0 International Public License CC-By.
The BAC X threat type interaction was significant, ηp2 = 0.08, b = −0.4, t(83) = 2.73, P = 0.008, such that the BAC effect on probe P3 suppression was significantly greater during uncertain (ηp2 = 0.07, b = −0.4, t(83) = 2.44, P = 0.017) than certain threats (ηp2 < 0.01, b = 0.0, t(83) = 0.18, P = 0.855). The quadratic BAC X threat type effect was not significant, ηp2 = 0.02, b = 0.0, t(82) = 1.16, P = 0.251. The BAC X threat type interaction for probe P3 suppression remained significant in GLMs that controlled for individual differences in either startle potentiation (ηp2 = 0.09, b = –0.4, t(82) = 2.80, P = 0.006) or self-reported anxiety (ηp2 = 0.10, b = –0.4, t(82) = 2.94, P = 0.004). No significant correlations were observed among the uncertain–certain difference scores for any of these three measures, probe P3 and startle potentiation (r = –0.01, P = 0.942), probe P3 and self-reported anxiety (r = 0.7, P = 0.487), and startle potentiation and self-reported anxiety (r = 0.13, P = 0.229). Post-hoc, exploratory analysis suggested the relationships among these dependent variables did not change across the varying BACs (see Supplementary Materials).
Discussion
Cues for both uncertain and certain threats elicited robust negative affective response (i.e. defensive reactivity, subjective anxious response) and increased attention among sober participants as indexed by startle potentiation, self-report and Probe P3, respectively. However, uncertain threats increased defensive reactivity and subjective anxiety more potently than certain threats. These observations join recent experimental and other evidence (Koolhaas et al., 2011; Bradford et al., 2013; Jackson et al., 2015) to indicate that uncertain threats are generally more affectively aversive and/or anxiogenic than certain threats. Nonetheless, it appears that cues for uncertain and certain threat recruited comparable attention resources as indicated by comparable suppression of attentional response to the startle probe during each threat cue relative to the no-shock cues (Nelson et al., 2015). In other words, even though uncertain threats prompt stronger negative affective response, all threats appear to increase attentional processing that may be critical to support adequate appraisal and subsequent adaptive behavioral response, at least among sober individuals. These data also highlight that the stimulus characteristics and neurobiological systems that drive affective response are at least partially separable from the characteristics that recruit increased attention (Berridge et al., 2009).
Decades of research broadly indicate that the affective ‘stress response dampening (SRD)’ properties of alcohol reinforce alcohol use among both recreational drinkers and alcoholics alike (Sher, 1987; Sayette, 2017). However, it remains important to clarify when, how and for whom alcohol SRD occurs to answer fundamental questions about this popular drug’s reinforcing effects and to mitigate the negative consequences associated with its excessive use (Bradford et al., 2013). We have now generated robust evidence that alcohol SRD is most potent ‘when’ the threats are characterized by some degree of uncertainty, regardless if this uncertainty concerns the threat’s location (this study), probability (Hefner and Curtin, 2012), timing (Hefner et al., 2013), combination of probability and timing (Moberg and Curtin, 2009), or intensity (Bradford et al., 2013). Thus, we have begun to speculate ‘how’ alcohol provides SRD, implicating neural mechanisms involving CRF and NE sensitive pathways in the central extended amygdala that selectively mediate startle potentiation to uncertain threats (Davis et al., 2010). Recently emerging theory and empirical evidence regarding ‘who’ may be most sensitive to alcohol SRD implicates the role of strong negative affective reinforcement among alcoholics with a history of chronic, heavy alcohol use (Kaye et al., 2017; Moberg et al., 2017).
To our knowledge, the current study provides the first evidence that alcohol dose-dependently disrupts attention during uncertain threats but not certain threats as indicated by decreased probe P3 suppression during uncertain but not certain threats. Equally important, this selective disruption in attentional processing appears to be independent of alcohol’s effects on negative affect. As such, the effect of alcohol on attention to uncertain threats may offer an additional source of reinforcement for drinking. Uncertain threats (e.g. academic or professional performance reviews, novel social interactions, many financial stressors) may make us anxious, but they can also occupy our limited attentional resources. Such perseverative rumination on these uncertain threats may interfere with adaptive flexible allocation of attention to other pressing decisions, tasks, or behaviors. To the degree that alcohol may interrupt this focus and release these attentional resources for other uses, ‘drinking to forget’ may be reinforcing and even adaptive in some select situations.
As we have described, alcohol’s effect on attention and negative affective response to uncertain threats may be reinforcing. However, these same two effects may also each contribute to maladaptive decision-making and/or risky behavior during uncertain threats. Clearly, adequate attention to and appraisal of threats are fundamental to good decision-making (Fernandes et al., 2013). Similarly, affect plays an important role in guiding adaptive behavior (Charpentier et al., 2016). The current study suggests that alcohol independently impairs both of these important decision-making processes when intoxicated individuals are presented with uncertain threats. For example, the inebriated drinker who considers driving home from the bar may not adequately fear or attend to the potential but uncertain negative consequences (arrest, injury to self or others). They may also not fully attend to and appraise their own level of intoxication or alternative options available to them to get home (e.g. government sponsored ‘safe-ride’ programs, public transportation, sober friends). Viewed through this lens, the decision to drive home drunk may seem less surprising though still clearly costly to both the drinker and society. These impairments in attention and/or negative affective response may individually or together with other processes (e.g. reduced inhibitory control; Weafer et al., 2014), help to explain many maladaptive or otherwise risky intoxicated behaviors observed under uncertain threats (e.g. sexual risk-taking, certain types of aggressive behavior, petty theft, excessive gambling at casinos). On the other hand, the current results suggest that attention and affective response to certain threats are somewhat immune to alcohol. Thus, intoxicated behavior may seem less inappropriate when the threats are imminent and highly probable (e.g. the respectful intoxicated driver cooperating with the police officer after being pulled over). Furthermore, education, law enforcement and public policy efforts that shift the balance of consequences from uncertain to more certain may also yield benefits to both drinkers and their community.
This study reinforces the importance of recent calls ‘to continue to develop methods for assessing cognitive and affective processes simultaneously’ in human drug research (Sayette, 2017). Many existing theoretical perspectives focus on alcohol’s effects on both affective and cognitive processes including attention, working memory and appraisal to account for its reinforcing effects, its impact on behavior and etiological mechanisms for alcohol use disorder (see Sayette, 2017 for review). However, these perspectives differ with respect to the presumed causal connections and ordering among these processes. Concurrent measurement of self-reported affect, startle potentiation, and probe P3 suppression in this study allowed us to conclude that alcohol has independent effects on negative affect and attention during presentation of threats rather than alterative mediated pathways that were possible but not consistent with our data. Yet other work using simultaneous measurement of cognitive and affective processing suggests alcohol’s effects on emotional responses may be mediated by other types of attentional processing (e.g. explicit focus of attention in complex visual environments; Curtin et al., 2001). Future research can strengthen conclusions about the independence or causal inter-connections among various affective and cognitive processes by including direct manipulations of attention, appraisal and working memory as well (e.g. Casbon et al., 2003). We also believe that future research on the cognitive-attentional effects of alcohol should continue to examine these processes in the context of affective or otherwise motivationally relevant stimuli as we do in this study. Previous research has often explored alcohol’s effects in traditional cognitive tasks (e.g. Stroop, Curtin and Fairchild, 2003; N-back, Casbon et al., 2003). While informative, alcohol’s most salient effects on decision-making and behavior occur in affectively-charged contexts.
In this study, we demonstrated that alcohol dose-dependently reduced negative affective response and disrupted attentional processing during threats by concurrent measurement of subjective anxious response, defensive reactivity and probe P3 suppression. Alcohol’s effects on affect and attention were limited to threats that were uncertain, with response during certain threats spared even at relatively high levels of intoxication. Furthermore, the effects of alcohol on negative affective response and attention appeared to occur via independent pathways. As such, we have identified two separate mechanisms that may both reinforce alcohol use but also account for maladaptive behavior in some situations.
Several aspects of this work deserve further scrutiny in future research. First, we did not explicitly measure volitional behavior in the current study. While affect and attentional processes surely influence behavior, future work can combine our methods with paradigms from behavioral economics and related disciplines to directly test the causal impact of these impairments on decision-making and ultimately behavior. The use of measures such as eye tracking or imbedded reaction time tasks may further clarify, in real time, participants’ explicit focus of attention during these tasks. While subcortical CRF and NE sensitive pathways in the central extended amygdala are implicated in alcohol’s selective dampening of uncertain startle potentiation, the neural mechanisms responsible for alcohol’s effects on cortical structures associated with probe P3 and other measures of attention are less well understood. Furthermore, previous work suggests that alcohol’s effects on uncertain startle potentiation are pharmacological in nature. Our use of a placebo manipulation but no ‘true’ no-alcohol condition did not allow full assessment of the relative contribution of potential expectancy and pharmacological effects that may have influenced attentional processing in the current study. Nonetheless, future research can clarify the neural circuits and neurotransmitter systems implicated in this study by more direct manipulation (e.g. administration of neurotransmitter agonists and antagonists, transcranial magnetic stimulation) or measurement (neuroimaging techniques including MEG, fMRI, PET).
This study extends other recent research to suggest that uncertainty, broadly defined, is an important characteristic of threats that modulates the degree of impairment produced by alcohol intoxication. Future research can examine these effects in more ecologically valid and socially relevant contexts such as in the presence of drinking partners and other social interactions (e.g. Fairbairn and Sayette, 2014; Sayette, 2017). In addition, other potentially important characteristics of threats such as their intensity and controllability should also be carefully considered in future research. Furthermore, we did not assess alcohol’s well documented effects on responses to appetitive stimuli (e.g. Berridge et al., 2009). It remains to be seen if alcohol’s interactions with uncertainty extend to responses to uncertain rewards or other appetitive stimuli. Research using tasks similar to the current study but with uncertain rewards is an important next step to fully characterize alcohol’s broad effects. Finally, the impact of policy changes targeting uncertainty reduction to reduce the societal costs of intoxicated behavior could be examined in the laboratory and field to evaluate policy effectiveness but also as further test of the influence of these mechanisms on behavior.
Supplementary Material
Acknowledgements
All authors contributed to development of the study concept and design. D. E. Bradford, C A. Motschman and M.J. Starr collected the data. D.E. Bradford and M.J. Starr performed the data analysis and interpretation under J. J. Curtin’s supervision. D. E. Bradford drafted the manuscript, and all other authors provided critical revisions. All authors approved the final manuscript.
Funding
NIH grant R01 AA024388 to J.J.C. provided funding for this project.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Alius M.G., Pané-Farré C.A., Löw A., Hamm A.O. (2015). Modulation of the blink reflex and P3 component of the startle response during an interoceptive challenge. Psychophysiology, 52, 140–8. [DOI] [PubMed] [Google Scholar]
- Benning S.D., Rozalski V., Klingspon K.L. (2015). Trait absorption is related to enhanced emotional picture processing and reduced processing of secondary acoustic probes. Psychophysiology, 52, 1409–15. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D.C., Griebel G., Pobbe R., Blanchard R.J. (2011). Risk assessment as an evolved threat detection and analysis process. Neuroscience and Biobehavioral Reviews, 35, 991–8. [DOI] [PubMed] [Google Scholar]
- Blumenthal T.D., Cuthbert B.N., Filion D.L., Hackley S., Lipp O.V., Van Boxtel A. (2005). Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42, 1–15. [DOI] [PubMed] [Google Scholar]
- Bradford D.E., Kaye J.T., Curtin J.J. (2014a). Not just noise: individual differences in general startle reactivity predict startle response to uncertain and certain threat. Psychophysiology, 51, 407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford D.E., Magruder K.P., Korhumel R.A., Curtin J.J. (2014b). Using the threat probability task to assess anxiety and fear during uncertain and certain threat. Journal of Visualized Experiments: JoVE, 91, e51905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford D.E., Shapiro B.L., Curtin J.J. (2013). How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science, 24, 2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Codispoti M., Lang P.J. (2006). A multi-process account of startle modulation during affective perception. Psychophysiology, 43, 486–97. [DOI] [PubMed] [Google Scholar]
- Brainard D.H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–6. [PubMed] [Google Scholar]
- Casbon T.S., Curtin J.J., Lang A.R., Patrick C.J. (2003). Deleterious effects of alcohol intoxication: diminished cognitive control and its behavioral consequences. Journal of Abnormal Psychology, 112, 476–87. [DOI] [PubMed] [Google Scholar]
- Charpentier C.J., De Neve J.-E., Li X., Roiser J.P., Sharot T. (2016). Models of affective decision making: how do feelings predict choice? Psychological Science, 27, 763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B.R., Echiverri A.M., Covington M.F., Grillon C. (2008). Modality-specific attention under imminent but not remote threat of shock: evidence from differential prepulse inhibition of startle. Psychological Science, 19, 615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J.J. (2011). PhysBox: the Psychophysiology toolbox. An open source toolbox for psychophysiological data reduction within EEGLab, Available: http://dionysus.psych.wisc.edu/PhysBox.htm.
- Curtin J.J. (2015). lmSupport: support for linear models Available: https://cran.r-project.org/web/packages/lmSupport/index.html.
- Curtin J.J., Fairchild B.A. (2003). Alcohol and cognitive control: implications for regulation of behavior during response conflict. Journal of Abnormal Psychology, 112, 424–36. [DOI] [PubMed] [Google Scholar]
- Curtin J.J., Patrick C.J., Lang A.R., Cacioppo J.T., Birbaumer N. (2001). Alcohol affects emotion through cognition. Psychological Science, 12, 527–31. [DOI] [PubMed] [Google Scholar]
- Davis M. (2006). Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist, 61, 741–56. [DOI] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology Review, 35, 105–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Fairbairn C.E., Sayette M.A. (2014). A social-attributional analysis of alcohol response. Psychological Bulletin, 140, 1361–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris C., Treat T.A., Viken R.J., McFall R.M. (2008). Perceptual mechanisms that characterize gender differences in decoding women’s sexual intent. Psychological Science, 19, 348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes O., Portugal L.C.L., Alves R.C.S., et al. (2013). How you perceive threat determines your behavior. Frontiers of Human Neuroscience, 7, 632.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari V., Bradley M.M., Codispoti M., Lang P.J. (2010). Repetitive exposure: brain and reflex measures of emotion and attention. Psychophysiology, 48, 515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Baas J.M., Pine D.S., et al. (2006). The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry, 60, 760–6. [DOI] [PubMed] [Google Scholar]
- Grupe D.W., Nitschke J.B. (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14, 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm A., Schupp H.T., Weike A.I. (2007). Motivational organization of emotions: autonomic changes, cortical responses, and reflex modulation In: Davidson R.J., Scherer K.R., Goldsmith H.H., editors. Handbook of Affective Sciences, Oxford, UK: Oxford University, pp. 187–211. [Google Scholar]
- Hefner K.R., Curtin J.J. (2012). Alcohol stress response dampening: selective reduction of anxiety in the face of uncertain threat. Journal of Psychopharmacology (Oxf), 26, 232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K.R., Moberg C.A., Hachiya L.Y., Curtin J.J. (2013). Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology, 122, 756–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F., Nelson B.D., Proudfit G.H. (2015). In an uncertain world, errors are more aversive: evidence from the error-related negativity. Emotion Wash DC, 15, 12–6. [DOI] [PubMed] [Google Scholar]
- Kaye J.T., Bradford D.E., Magruder K.P., Curtin J.J. (2017). Probing for neuroadaptations to unpredictable stressors in addiction: translational methods and emerging evidence. Journal of Studies on Alcohol and Drugs, 78, 353–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A., Bradley M.M., Junghöfer M., Russmann T., Lowenthal W., Lang P.J. (2007). Cross-modal attention capture by affective stimuli: evidence from event-related potentials. Cognitive, Affective, and Behavioral Neuroscience, 7, 18–24. [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M., Bartolomucci A., Buwalda B., et al. (2011). Stress revisited: a critical evaluation of the stress concept. Neuroscience Biobehavioral Review, 35, 1291–301. [DOI] [PubMed] [Google Scholar]
- Linden D.E.J. (2005). The p300: where in the brain is it produced and what does it tell us? Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 11, 563–76. [DOI] [PubMed] [Google Scholar]
- MATLAB and Statistics Toolbox (2013). Natick, MA: The Mathworks, Inc.
- Moberg C.A., Bradford D.E., Kaye J.T., Curtin J.J. (2017). Increased startle potentiation to unpredictable stressors in alcohol dependence: possible stress neuroadaptation in humans. Journal of Abnormal Psychology, 126, 441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg C.A., Curtin J.J. (2009). Alcohol selectively reduces anxiety but not fear: startle response during unpredictable vs. predictable threat. Journal of Abnormal Psychology, 118, 335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B.D., Hajcak G. (2017). Defensive motivation and attention in anticipation of different types of predictable and unpredictable threat: a startle and event-related potential investigation. Psychophysiology, 54, 1180–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B.D., Hajcak G., Shankman S.A. (2015). Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology, 52, 887–94. [DOI] [PubMed] [Google Scholar]
- Pelli D.G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision, 10, 437–42. [PubMed] [Google Scholar]
- Picton T.W., Bentin S., Berg P., et al. (2000). Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology, 37, 127–52. [PubMed] [Google Scholar]
- R Development Core Team. (2015). R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. Available: http://www.R-project.org.
- Robinson O.J., Bond R.L., Roiser J.P. (2015). The impact of threat of shock on the framing effect and temporal discounting: executive functions unperturbed by acute stress? Frontiers of Psychology, 6, 1315.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio: Integrated development environment for R. (2016). Boston, MA: RStudio. Available: http://www.rstudio.org/.
- Sacks J.J., Gonzales K.R., Bouchery E.E., Tomedi L.E., Brewer R.D. (2015). 2010 national and state costs of excessive alcohol consumption. American Journal of Preventive Medicine, 49, e73–9. [DOI] [PubMed] [Google Scholar]
- Sayette M.A. (2017). The effects of alcohol on emotion in social drinkers. Behaviour Research and Therapy, 88, 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbrodt F.D., Maier M., Heene M., Zehetleitner M. (2015). Voluntary commitment to research transparency. Available: http://www.researchtransparency.org.
- Schupp H.T., Cuthbert B., Bradley M.M., Birbaumer N., Lang P.J. (1997). Probe P3 and blinks: two measures of affective startle modulation. Psychophysiology, 34, 1–6. [DOI] [PubMed] [Google Scholar]
- Sher K.J. (1987). Stress response dampening In: Blane H.T., Leonard K.E., editors. Psychological Theories of Drinking and Alcoholism, New York, NY: Guilford Press, pp. 227–71. [Google Scholar]
- Simmons J.P., Nelson L.D., Simonsohn U. (2012). A 21 Word Solution. Available: 10.2139/ssrn.2160588. [DOI]
- Weafer J., Mitchell S.H., de Wit H. (2014). Recent translational findings on impulsivity in relation to drug abuse. Current Addiction Reports, 1, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.