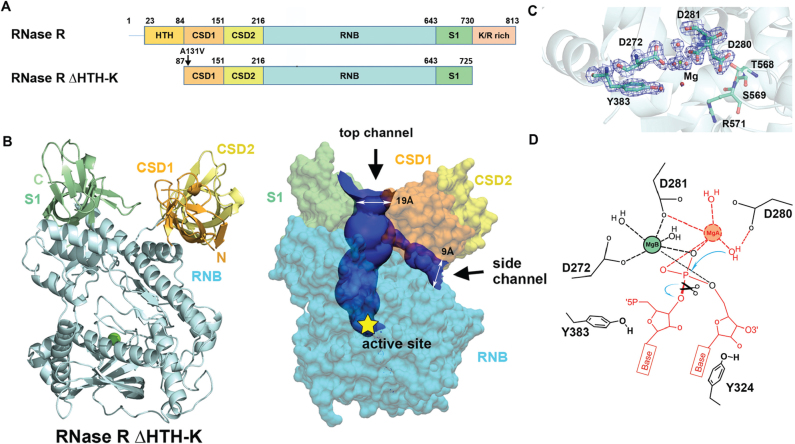
Figure 2.
Crystal structure of RNase R ΔHTH-K reveals two open channels and a Mg2+-bound active site. (A) Domain structures of the full-length RNase R and the truncated RNase R ΔHTH-K. (B) Crystal structure of RNase R ΔHTH-K reveals that the RNB domain (sky blue) is capped with S1 (light green), CSD1 (orange yellow) and CSD2 (yellow) domains (PDB entry: 5XGU). The Mg2+ located in the active site is shown as a green sphere. The top and side RNA-binding channels displayed in the right panel were calculated by HOLE2 (39). (C) The 2Fo - Fc Fourier map of the active site in the RNB domain contoured at 1.9σ (blue). The Mg (B) ion is displayed as a green sphere, whereas water molecules are displayed as red spheres. (D) The proposed two-metal-ion-dependent hydrolysis mechanism of RNase R. Only one Mg2+ (B site) that bound to D272 and D281 is observed in the crystal structure. The second Mg2+ (A site) is only bound upon RNA binding. D280 functions as the general base to deprotonate the Mg(A)-coordinated water for nucleophilic attack on a scissile phosphate. The RNA and Mg2+ atoms displayed in red are not observed in the crystal structure of the apo-form of RNase R.