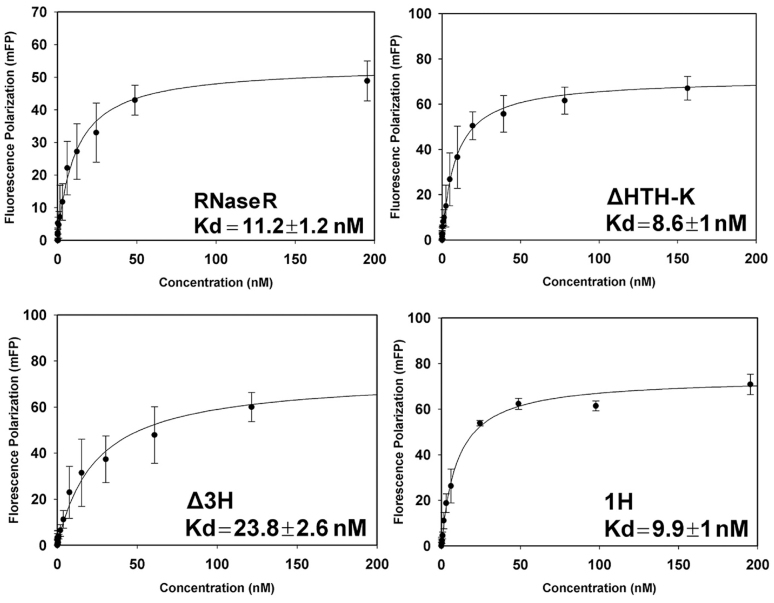
Figure 4.
RNA-binding affinities of RNase R mutants measured by fluorescence polarization assays. A 5′-end Cyanine-3-labeled single-stranded RNA (30 nucleotides, polyA) was used as the substrate to measure the binding affinity with RNase R proteins in the presence of EDTA. The ssRNA binds RNase R with a Kd of 11.2 ± 1.2 nM for the full-length RNase R, 8.6 ± 1.0 nM for RNase R ΔHTH-K, 23.8 ± 2.6 nM for RNase R Δ3H, and 9.9 ± 1.0 nM for RNase R 1H. The average of three independent experiments is shown with error bars representing one standard deviation. Kd values with standard errors were obtained by fitting the curve to a one-site saturation-binding model using SigmaPlot (34).