Abstract
2-Methylthio-N6-isopentenyl modification of adenosine (ms2i6A) is an evolutionally conserved modification that is found in transfer RNAs (tRNAs). We have recently shown that Cdk5 regulatory subunit-associated protein 1 (Cdk5rap1) specifically converts i6A to ms2i6A at position A37 of four mitochondrial DNA-encoded tRNAs, and that the modification regulates efficient mitochondrial translation and energy metabolism in mammals. Curiously, a previous study reported that ms2i6A is present abundantly in nuclear-derived RNA species such as microRNAs, but not in tRNA fractions. To fully understand the molecular property of ms2i6A, the existence of non-canonical ms2i6A must be carefully validated. In the present study, we examined ms2i6A in total RNA purified from human and murine ρ0 cells, in which mitochondrial DNA-derived tRNAs were completely depleted. The ms2i6A was not detected in these cells at all. We generated a monoclonal antibody against ms2i6A and examined ms2i6A in murine RNAs using the antibody. The anti-ms2i6A antibody only reacted with the tRNA fractions and not in other RNA species. Furthermore, immunocytochemistry analysis using the antibody showed the predominant localization of ms2i6A in mitochondria and co-localization with the mitochondrial elongation factor Tu. Taken together, we propose that ms2i6A is a mitochondrial tRNA-specific modification and is absent from nuclear-encoded RNA species.
INTRODUCTION
In all organisms, transfer RNA (tRNA) undergoes various post-transcriptional modifications (1). To date, more than 100 species of tRNA modifications have been reported in all three domains of life (2). Most modifications have been found in bases near the anticodon region, particularly at positions 34 and 37 (3). These modifications facilitate correct codon-anticodon base-pairing, thus promoting efficient protein translation (3).
2-Methylthioation modification is an evolutionally conserved modification found across species (2). In mammalian cells, there are two forms of 2-methylthiolation: 2-methylthio-N6-threonylcarbamoyadenosine (ms2t6A) and 2-methylthio-N6-isopentenyladenosine (ms2i6A) (2). Cdk5 regulatory subunit associated protein 1-like 1 (Cdkal1) converts t6A to ms2t6A at position A37 of cytosolic tRNALys(UUU) in mammalian cells, with a profound impact on both molecular and physiological functions (4,5). A deficiency of ms2t6A impairs the accurate translation of the Lys codons, resulting in the production of aberrant proinsulin and the induction of aberrant glucose metabolism (5). Importantly, genetic variants of CDKAL1 have been associated with the development of type 2 diabetes in humans (6). Individuals carrying risk CDKAL1 mutations exhibit a reduction of ms2t6A modification levels, which is associated with a decrease in insulin secretion (7–9).
Cdk5 regulatory subunit-associated protein 1 (Cdk5rap1) is a homolog of Cdkal1 in mammalian cells (4). Cdk5rap1 contains a mitochondria-targeting sequence at the N terminus that guides the enzyme to the inner membrane of mitochondria (10). Cdk5rap1 converts i6A37 to ms2i6A37 in four mitochondrial DNA-encoded tRNAs, mt-tRNATrp, mt-tRNATyr, mt-tRNAPhe and mt-tRNASer(UCN) (10). Similar to ms2t6A, ms2i6A is important for efficient and accurate translation in mitochondria. In Cdk5rap1-null mice, the absence of ms2i6A decreases the translation of mitochondrial DNA-derived respiratory subunits and impairs electron transport and aerobic respiration (10). Consequently, the cardiac function and skeletal muscle function of Cdk5rap1-null mice are significantly impaired due to insufficient energy metabolism. Importantly, the ms2i6A levels in these mt-tRNAs are substantially decreased in patients with mitochondrial disease. These results strongly suggested that ms2i6A modification of mt-tRNAs is crucial for the mitochondrial translation and that the disruption of ms2i6A modification is a key element of the molecular pathogenesis of the mitochondrial diseases.
While our results have clearly shown that Cdk5rap1-mediated ms2i6A modification occurs in mitochondrial DNA-encoded mt-tRNAs (10), a previous study reported that ms2i6A might exist in nuclear-encoded RNA species (11). Reiter et al. fractionated the total RNA of HeLa cells into tRNA, small RNA, polyA-RNA and ribosomal RNA (rRNA) fractions and examined ms2i6A modification using mass spectrometry. Surprisingly, the ms2i6A modification was almost absent from the tRNA fraction. The ms2i6A modification was rather highly enriched in the miRNA and the poly-A RNA fractions. The authors hypothesized that the non-canonical ms2i6A modification in nuclear-encoded RNA species might be catalyzed by a splicing variant of CDK5RAP1 that lacks the mitochondria-targeting sequence. These findings challenged the current understanding of ms2i6A modification of the exclusive occurrence of ms2i6A modification in mt-tRNAs, and raised a possibility that the ms2i6A modification might control cellular functions through nuclear-encoded RNA species instead of mitochondrial tRNAs.
Does ms2i6A exist in nuclear-encoded RNA specises? To answer this question, it is necessary to examine the presence of ms2i6A in such RNAs using carefully designed experimental approaches. It should be noted that, in growing cells, ∼80–90% of total RNA is rRNA, and 10–15% is tRNA (12). By contrast, mRNA constitutes 3–7% of total RNA, and miRNA constitutes only 0.003–0.02% of total RNA (12). Therefore, the biochemical purification of miRNA or mRNA without the contamination of mitochondrial DNA-derived tRNA is highly challenging during the validation of ms2i6A modification in individual RNA species.
In the present study, we carefully investigated the presence of ms2i6A modification in nuclear-encoded RNA species using cell biological approaches. We provide evidence that the ms2i6A modification does not exist in nuclear-encoded RNA species.
MATERIALS AND METHODS
Animals
Cdk5rap1 knockout (KO) mice were generated and maintained as described previously (10). Littermates of wild-type (WT) and KO mice (8–12 weeks old) were used for experiments unless otherwise specified. Animals were housed at 25°C with 12-h light and 12-h dark cycles. All the animal procedures were approved by the Animal Ethics Committee of Kumamoto University, Japan (Approval ID: A29–016-163).
Cell culture
HeLa cells and B82 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) medium supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. HeLa cells and B82 cells devoid of endogenous mitochondrial genomes (HeLa ρ0 cells and B82 ρ0 cells) were kindly provided by Dr Kazuto Nakata (Tsukuba University). HeLa ρ0 cells and B82 ρ0 cells were cultured in DMEM medium (Invitrogen) supplemented with 10% FBS, pyruvic acid (Invitrogen, final concentration 10 μM) and uridine (Sigma, final concentration 100 μg/m). Hybridoma cells that produce ms2i6A antibody were cultured in GIT medium (Wako) at 37°C and 5% CO2.
RNA purification
Total RNA was isolated using TRIzol (Invitrogen) following the manufacturer’s instructions. mRNA was purified using Oligotex-dT(30) mRNA purification kit (TAKARA) following the manufacturer’s instruction. The eluted mRNA was further subjected to selection for large size (>200 nt) RNA using RNA Clean & Concentrator (Zymo Research). Size selection of mRNA was repeated twice in order to achieve maximum elimination of small RNA contamination.
Gene expression analysis
First-strand cDNA synthesis from total RNA was performed using the PrimeScript RT reagent Kit (TAKARA). Real-time polymerase chain reaction (PCR) quantitative analysis was performed using SYBR premix Taq (TAKARA) and the 7300 Real-Time PCR System (Applied Biosystems) following the manufacturer’s instructions. For total RNA from HeLa ρ0 cells and B82 ρ0 cells, cDNA was synthesized using the Transcriptor First-Stand cDNA Synthesis Kit (Roche Diagnostics) with reverse primer targeting mt-tRNAPhe, followed by quantitative PCR using forward and reverse primers as following:
mouse mt-tRNAPhe:
forward: 5′-GCTTAATAACAAAGCAAAGCA
reverse: 5′-TATCCATCTAAGCATTTTCA
human mt-tRNAPhe
forward: 5′-CTCCTCAAAGCAATACACTG
reverse: 5′-AGCCCGTCTAAACATTTTCA
mouse mt-tRNASer(UCN):
forward: 5′- CATATAGGATATGAGATTGGC
reverse: 5′- AACCCCCTAAAATTGGTTTCA
Modification analysis by mass spectrometry
Twenty microliters of total RNA isolated from HeLa cells, B82 cells, HeLa ρ0 cells and B82 ρ0 cells were mixed with 1.5 μl of P1 Nuclease (WAKO), 1 μl of alkaline phosphatase (TAKARA) and 2.5 μl of 200 mM HEPES (pH 7.0), and the mixture was incubated at 37°C for 3 h to completely digest RNA. The digestion products were separated on a C18 reverse phase column (GL Science), and i6A, ms2i6A and adenosine (A) were measured using a mass spectrometer (Agilent 6460) as described previously (13).
Purification of ms2i6A antibody
Synthetic ms2i6A was used to generate monoclonal antibody (ITM Co., Ltd. Japan). A hybridoma clone was established using a standard method described elsewhere. Hybridoma cells secreting the ms2i6A antibody were cultured in 50-ml flasks until confluent. Thereafter, 10 ml of the culture supernatant was added to a Protein-G column (GE Healthcare), and the antibody was adsorbed onto the column. Thereafter, the antibody was eluted using an elution buffer contained in the MAbTrap Kit (GE Healthcare). Finally, the eluted antibody was added to a centrifugal filter (Amicon Ultra-15, Millipore), and buffer exchange and concentration determination were performed using phosphate-buffered saline (PBS), followed by storage at −80°C until use.
ELISA assay
Competitive ELISA was used to validate the specificity of the ms2i6A antibody. Briefly, a 96-well plate was coated with anti-mouse Fc (Sigma) at a final concentration of 5 μg/ml at 4°C overnight. The plate was washed with PBS three times and was used for ELISA immediately. Anti-ms2i6A was diluted (1:100) with a blocking solution (1% Block Ace, DS Pharma Biomedical), and 50 μl of the diluted antibody solution was added to the 96-well plate. Next, vehicle, ms2i6A, i6A or m1A was diluted with the blocking solution to 10 μg/ml, and 50 μl of the diluted compound was added to the 96-well plate. Finally, horseradish peroxidase-conjugated ms2i6A was diluted with the blocking solution and added to the plate. The plate was incubated at room temperature for 2 h, followed by washing with PBS containing 0.1% Tween 20. O-phenylenediamine dihydrochloride (Sigma) solution was added to each well for the reaction with HRP. The reaction was stopped with 1 M phosphoric acid solution. A plate reader (WAKO) was used for the examination of the absorbance at OD492 nm.
Dot blotting
Total RNA derived from WT mice and total RNA derived from Cdk5rap1 KO mice (1 μg/μl) were added dropwise (2 μl each) to an Amersham Hybond-N + membrane (GE Healthcare), crosslinked with ultraviolet light and washed with 0.05% PBST. Blocking was carried out using the blocking solution (1% Block Ace, DS Pharma Biomedical) for 1 h. Thereafter, ms2i6A antibody (0.05 mg/ml) was diluted with the blocking solution (1:200) and incubated with the membrane at 4°C overnight. The next day, after washing 3–4 times with PBST, the secondary antibody (anti-mouse HRP) (1:1000 dilution) was added and incubated with the membrane for 1 h. Finally, the ECL Prime (GE Healthcare) reagent was added, and imaging was performed using an ImageQuant 400 Transilluminator (GE Healthcare).
Isolation of mitochondria
Mitochondria were isolated from mouse liver as described previously (10). Briefly, the liver was gently homogenized in a homogenization buffer (225 mM mannitol, 75 mM sucrose, 10 mM HEPES-NaOH at pH 7.6, 2 mM ethylenediaminetetraacetic acid). Next, the supernatant (supernatant A) was recovered by centrifugation at 800 × g for 10 min at 4°C. The supernatant was centrifuged at 7500 × g for 10 min at 4°C to obtain the crude mitochondrial fraction. The crude mitochondria were overlaid on a discontinuous gradient consisting of 1.5 M and 1 M sucrose, followed by centrifugation at 15 700 rpm at 4°C for 60 min. The purified mitochondrial fraction was homogenized in TRIzol to extract mitochondria-derived total RNA.
Northern blotting
Total RNA was purified from mouse liver and mitochondria isolated from mouse liver with TRIzol (Invitrogen) and then separated on a 6% TBE-Urea gel (Invitrogen). RNA was visualized by staining with SYBR Gold (Invitroge). The RNA was then transferred to an Amersham Hybond-N+ membrane (upward capillary transfer) using the conventional capillary transfer method described elsewhere. Next, anti-ms2i6A was added to the membrane and incubated at 4°C overnight. The ECL Prime Western Blotting Detection Reagent (GE Healthcare) was used to visualize signals corresponding to ms2i6A.
Fluorescent immunostaining
HeLa cells (0.75 × 105 cells/ml) were seeded in a glass-bottomed dish (IWAKI) and were cultured overnight. The following day, control siRNA (siControl) and siRNA against Cdk5rapl (siCdk5rapl) were transfected and cultured for 2 days. Next, the cells were incubated with Mitotracker Red (Molecular Probe, final concentration: 100 pM) for 30 min and then were fixed with 4% paraformaldehyde (WAKO). Cells were washed with PBS and blocked with 4% bovine serum albumin. The ms2i6A antibody was added (1:100 dilution) and reacted overnight at 4°C. The next day, Alexa488-conjugated anti-mouse secondary antibody (1: 100 dilution, Molecular Probe) was further added to the cells. Images were observed with a confocal laser scanning microscope (Olympus, FV1000). To stain the mitochondrial protein translation machinery, TUFM antibody (Abcam, 1:200) was used.
RESULTS
Absence of ms2i6A modification from human and murine Rho0 (ρ0) cells
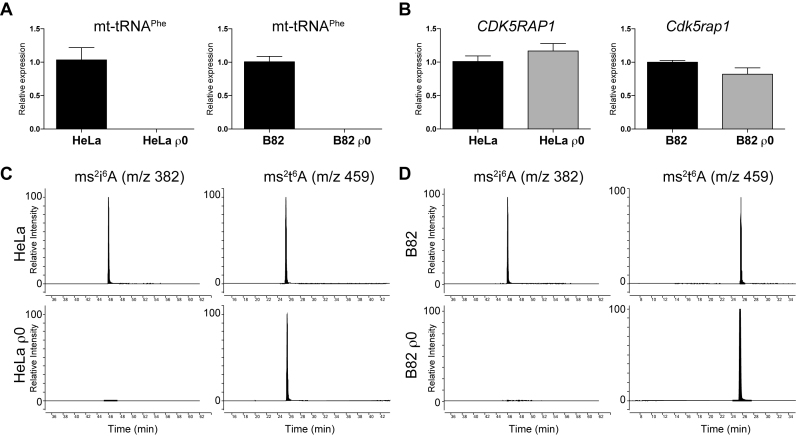
Rho0 cells (ρ0 cells) are biochemically engineered cells, in which mitochondrial DNA are depleted completely by chemical compounds. Thus the cells do not contain mitochondrial transcripts but the nuclear-derived RNA species remain intact (14). Examination of ρ0 cell-derived total RNA would clearly verify the presence of ms2i6A derived from nuclear-encoded RNA species, if it is indeed present in the RNA species, without the contamination of ms2i6A-containing mitochondrial DNA-derived tRNAs. We isolated total RNA from human-derived HeLa cells and HeLa ρ0 cells, as well as mouse-derived B82 cells and B82 ρ0 cells, and examined whether ms2i6A-containing mt-tRNA (mt-tRNAPhe) was depleted in the ρ0 cells by quantitative PCR. As expected, the levels of ms2i6A-containing mt-tRNAPhe in HeLa ρ0 cells and B82 ρ0 cells were below the detection limit compared with those in control cells (Figure 1A). In contrast to mt-tRNAs, the expression levels of nuclear-encoded CDK5rap1 were compatible between ρ0 cells and control cells (Figure 1B). Given the successful validation of ρ0 cells, we then subjected total RNA to mass spectrometry analysis to examine the presence of ms2i6A (Figure 1C and D). The ms2i6A was clearly absent from the total RNA of HeLa ρ0 cells and B82 ρ0 cells. As a control, we examined ms2t6A modification that was derived from nuclear-encoded cytosolic tRNALys(UUU), and found that ms2t6A modification remained intact in all cells (Figure 1C and D). These results demonstrated the absence of ms2i6A in nuclear-derived RNA species.
Figure 1.
Analysis of ms2i6A in ρ0 cells. (A) Quantification of mt-tRNAPhe in the total RNA of HeLa cells, HeLa ρ0 cells, B82 cells and B82 ρ0 cells. n = 3 each. Data are the mean ± s.e.m. (B) Quantification of Cdk5rap1 transcripts in the total RNA of HeLa cells, HeLa ρ 0 cells, B82 cells and B82 ρ0 cells. n = 3 each. Data are the mean ± s.e.m. (C and D) Mass spectrometry analysis of ms2i6A and ms2t6A in the total RNA of HeLa cells (C), HeLa ρ0 cells (C), B82 cells (D) and B82 ρ0 cells (D).
Generation of ms2i6A antibody
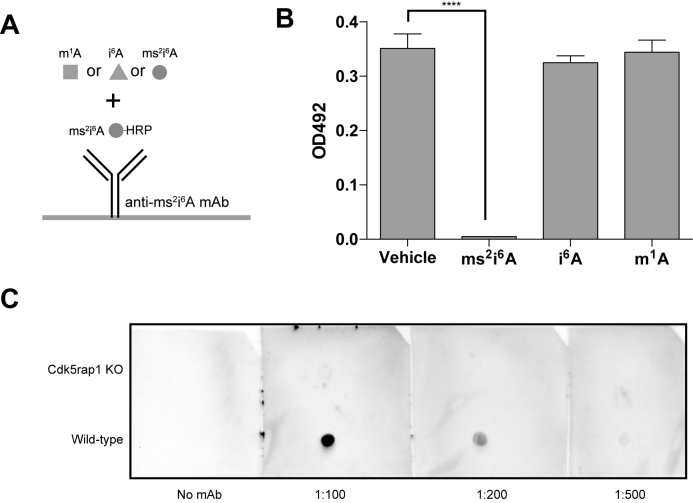
In addition to mt-tRNAs, mitochondrial DNA encodes two genes for mt-rRNAs and 13 genes for mt-mRNAs (10). There is a possibility that the ms2i6A modification might be present in mt-rRNA and mt-mRNA. Given the technical difficulty of purifying individual mt-rRNA and mt-mRNAs without the contamination of mt-tRNA, we sought to separate mt-tRNA from other mt-tRNA species by denaturing gel and then to detect ms2i6A using specific antibodies. We generated a monoclonal antibody by immunizing mice with synthetic ms2i6A. Competitive ELISA was used to validate the specificity of the ms2i6A antibody (Figure 2A). The synthetic ms2i6A abolished HRP-conjugated ms2i6A interaction with the antibody, whereas the synthetic i6A or methylated adenosine (m1A) had no effect on this interaction (Figure 2B), suggesting that the antibody specifically recognizes ms2i6A. To examine whether the antibody can recognize ms2i6A in intact RNA, the total RNA of WT and Cdk5rap1 KO mice was spotted on a membrane, fixed by UV irradiation and was then subjected to detection by conventional western blotting. The ms2i6A antibody reacted nicely with the total RNA of WT mice but not with that of Cdk5rap1 KO mice (Figure 2C). These results suggest that the antibody could detect the ms2i6A modification in intact RNA.
Figure 2.
Validation of the ms2i6A antibody. (A) Schematic illustration of competitive ELISA for the validation of the ms2i6A antibody. (B) The ms2i6A antibody specifically recognizes ms2i6A but not the derivatives i6A and m1A. n = 3 for each. Data are the mean ± s.e.m. ****P < 0.0001 by Student's t-test. (C) Dot blot analysis of ms2i6A in the total RNA purified from the liver tissues of WT mice or Cdk5rap1 KO mice.
ms2i6A was undetectable in mt-mRNA and mt-rRNA
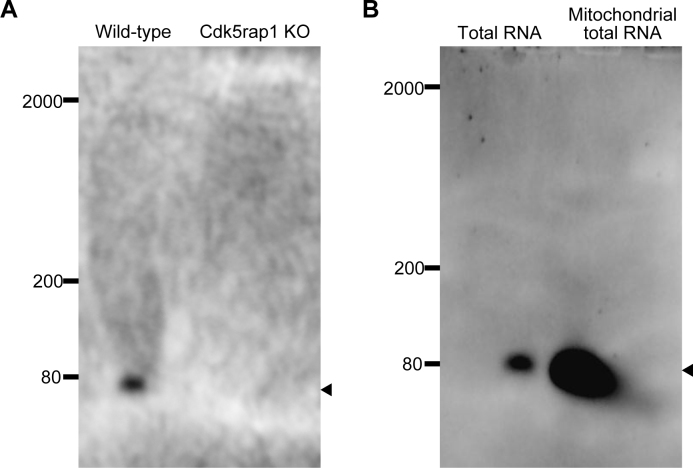
The specificity of the ms2i6A antibody prompted us to examine the presence of ms2i6A in each RNA species. Total RNA was extracted from the liver tissues of the WT and the Cdk5rap1 KO mice, followed by size-separation using denaturing Urea-TBE gel electrophoresis. In the total RNA of WT mice, an apparent single band was detected at 70–80 nt, which corresponded to the size of mt-tRNAs (Figure 3A). By contrast, no band was detected in the total RNA of Cdk5rap1 KO mice (Figure 3A). If mt-rRNA and/or mt-mRNA contain the ms2i6A modification, the antibody would detect the signals in the higher molecular weight region. However, no bands were observed in the molecular weight region of >80 nt in either WT or Cdk5rap1 KO mice (Figure 3A).
Figure 3.
Analysis of ms2i6A modification by northern blotting. (A) Total RNA purified from the liver of WT mice and Cdk5rap1 KO mice was separated by TBE-Urea gel electrophoresis, and then subjected to northern blotting with the ms2i6A antibody. (B) Total RNA from the liver of WT mice and mitochondrial total RNA purified from isolated mitochondria from the liver of WT mice were subjected to northern blotting using the ms2i6A antibody. Arrowheads indicate the position corresponding to tRNA.
The ms2i6A modification in mRNA might be difficult to detect due to the limited amount of mRNA in the total RNA fraction. We thus enriched mRNA from mouse liver total RNA using oligo(dT)-mediated affinity purification, followed by two rounds of size selection for RNA species with more than 200 nt in length (Supplementary Figure S1A). The rigorous purification resulted in a marked depletion of small size RNA species including tRNA, 5S rRNA and 5.8 rRNA (Supplementary Figure S1A). Indeed, cytosolic tRNALys in mRNA-enriched fraction was reduced to ∼0.9% after purification (Supplementary Figure S1B and C). Surprisingly, ms2i6A-containing mt-tRNASer(UCN) in mRNA-enriched fraction only reduced to 23.8%, despite the apparent elimination of tRNA as observed in the denaturing gel (Supplementary Figure S1B and C). After transferring RNA to membrane, we applied the anti-ms2i6A antibody to detect the modification in mRNA-enriched fraction. However, no signals were observed in the mRNA region in both total RNA and mRNA-enriched fractions. In contrast, ms2i6A signals were clearly detected at the size corresponding to tRNA in both fractions (Supplementary Figure S1D).
The number of mitochondrial DNA-derived RNA is far less than that of nuclear-derived RNA species, the sensitivity might be insufficient when the total RNA or mRNA-enriched fractions was subjected to detection by our antibody. To enhance the sensitivity of detection, we isolated mitochondrial total RNA from a purified mitochondria fraction, and applied anti-ms2i6A antibody to the fractions. Compared with the total RNA fraction, a very strong band in the mitochondrial total RNA fraction was detected at ∼80 nt, indicating the successful concentration of mt-tRNAs. Notably, no other band was detected even in this highly purified mt-RNA fraction (Figure 3B). These results suggested that ms2i6A exists only in mt-tRNA and not in other RNA species.
ms2i6A-modified mt-tRNA is localized in the vicinity of the mitochondrial translation machinery
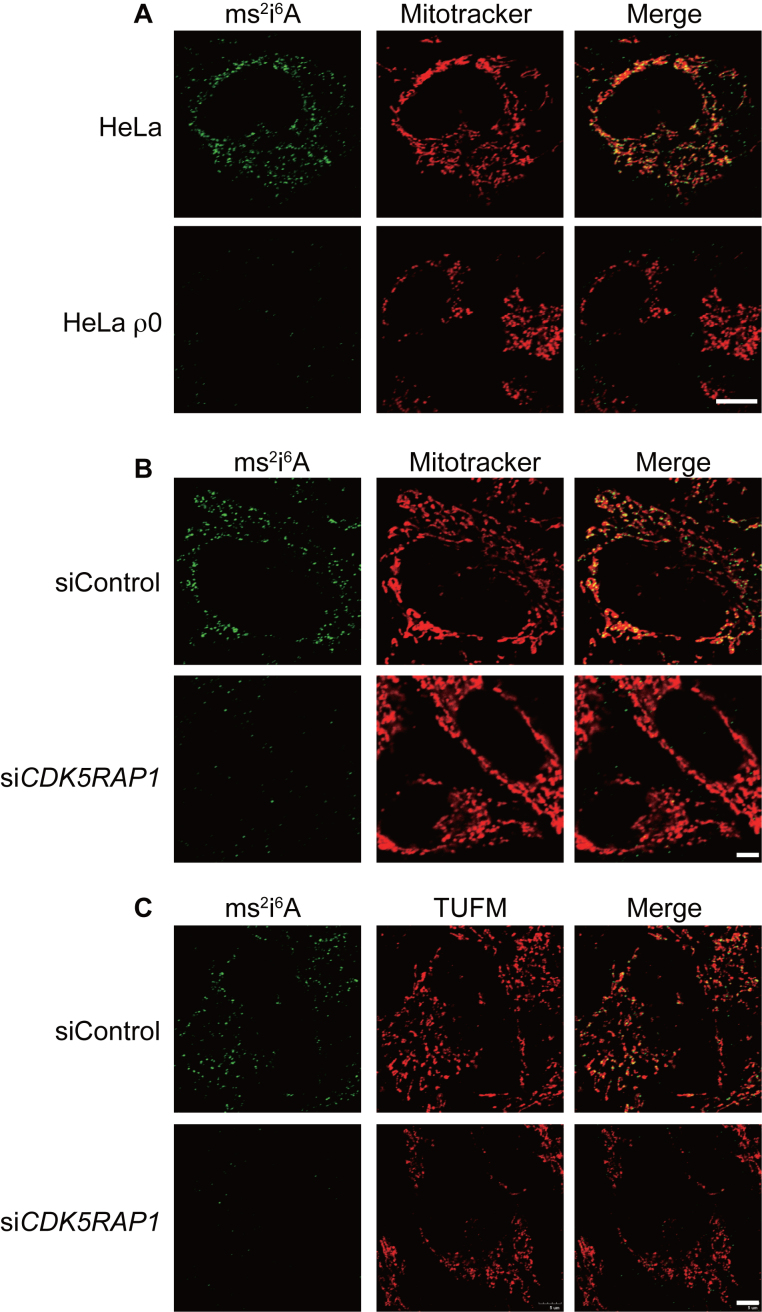
The superior specificity of the ms2i6A antibody prompted us to investigate the cellular localization of the ms2i6A modification by immunostaining. HeLa cells and HeLa ρ0 cells were stained with the antibody in the presence of Mitotracker. The spotty signals stained by the anti-ms2i6A antibody were nicely co-localized with Mitotracker in HeLa cells but had disappeared from HeLa ρ0 cells (Figure 4A). In addition, the mitochondrial localization of ms2i6A modification was diminished in HeLa cells when transfected with siRNA against Cdk5rap1 (Figure 4B), which further supports the idea that the modification occurs in tRNAs in mitochondria. Finally, we stained HeLa cells with the ms2i6A antibody in combination with antibody against mitochondrial elongation factor TUFM. The ms2i6A modification showed strong co-localization with TUFM (Figure 4C). Taken together, these results suggested that the ms2i6A modification exists in mt-tRNA, but not in the nuclear-derived RNA species.
Figure 4.
Immunostaining of cells with the ms2i6A antibody. (A) HeLa cells and HeLa ρ0 cells were stained with Mitotracker and the ms2i6A antibody. Note that ms2i6A was co-localized with Mitotracker. Bar = 10 μm. (B) HeLa cells were transfected with control siRNA (siControl) or siRNA against CDK5RAP1. Cells were stained with Mitotracker and the ms2i6A antibody. Bar = 5 μm. (C) HeLa cells were transfected with control siRNA (siControl) or siRNA against CDK5RAP1. Cells were stained with anti-TUFM and anti-ms2i6A. Bar = 5 μm.
DISCUSSION
The ms2i6 modification of adenosine in mt-tRNAs is critical for metabolism and energy expenditure in mammals (10). Deficiency of the modification causes malfunction of energy-consuming tissues, such as heart and skeletal muscle, and leads to the development of mitochondrial disease. Given the important role of the ms2i6A modification, the molecular property of the modification needs to be precisely understood.
Our data presented in this study provided strong supports to the idea that ms2i6A is absent in nuclear-encoded RNA species. Using mass spectrometric analysis, we confirmed that in both human and mouse-derived ρ0 cells, the ms2i6A was not detected in the intact nuclear-derived RNA. Furthermore, we generated a specific antibody against the ms2i6A and used two different methods to investigate the modified bases in RNA species. Immuno-blotting with the antibody gave a specific band at the size of tRNA and the signal of the band was significantly stronger with mitochondrial RNA fraction than total RNA fraction. Immunostaining of cells with the antibody showed numerous punctuated foci that were localized to mitochondria and TUFM. These results thus provided strong evidence that the ms2i6A modification is not present in nuclear-derived RNA species, at least in the samples used in this study. The defective metabolism observed in Cdk5rap1 KO mice (10) should solely be due to the defective mt-tRNA modification.
Mammalian cells contain multiple CDK5RAP1 variants due to alternative splicing (11,15). Interestingly, one of CDK5RAP1 variants, namely CDK5RAP1_v2, lacks N-terminus region that corresponds to mitochondria-targeting signal and is capable to localize in cytosol (11). Reiter et al. suggested that CDK5RAP1_v2 might be responsible for the ms2i6A modification of nuclear-derived RNA species (11). However, the cytosolic CDK5RAP1_v2 not only lacks mitochondria-targeting signal, but also lacks an important cysteine residue in the UPF0004 domain (10). Because this cysteine residue is absolutely required for the enzyme activity of CDK5RAP1 (10), it is likely that the cytosolic CDK5RAP1_v2 cannot modify any RNA species. Indeed, we have previously shown that the absence of ms2i6A modification in cells derived from Cdk5rap1 KO mouse was not rescued by overexpressing CDK5RAP1_v2 (10).
Why did Reiter et al. observed such a large amount of ms2i6A modification in nuclear-derived microRNA? Reiter et al. purified microRNA using a commercially available kit (11). However, it appears to be technically difficult to separate tRNA (∼80 nt) from microRNA (∼30 nt) using the kit as advised in the manufacturer's instructions. In fact, the size of microRNA purified by Reiter et al. was also almost identical to the size of tRNA (∼80 nt) (11). It is thus conceivable that the substantial amount of ms2i6A-containing mt-tRNA was co-purified with microRNA by the kit. Recently, tRNA-derived fragments (tRFs) have been emerging as a new group of functional small RNAs (16). These tRFs are ∼30 nt in length, which is the similar size of the conventional microRNAs. The tRFs can be generated through partial cleavage of full-length cytosolic tRNA and mt-tRNAs, which are widespread in cells and tissues (16). Thus, it is conceivable that the mt-tRNA-derived tRFs might also be co-purified by the microRNA purification kit. Therefore, the ms2i6A-containing microRNAs detected by Reiter et al. were most likely contaminated ms2i6A-containing mt-tRNA or mt-tRNA-derived fragments.
In addition to microRNA, Reiter et al. observed a substantial amount of ms2i6A modification in mRNA fraction. It is likely that the ‘ms2i6A-containing mRNA’ was also the contaminated ms2i6A-containing mt-tRNA. Indeed, a substantial amount of mt-tRNASer(UCN) remained in mRNA-enriched fraction even after extensive selection (see Supplementary Figure S1). It is worthwhile to mention that the signal of ms2i6A in mRNA-enriched fraction was compatible to that in total RNA fraction despite the apparent decrease of tRNA in mRNA-enriched fraction (See gel image in Supplementary Figure S1). Dense and large amount of tRNA in total RNA fraction might have reduced the accessibility of anti-ms2i6A antibody to mt-tRNA.
The unique structure of mt-tRNA might be one of the reasons that cause the selective contamination of mt-tRNA during mRNA purification. It is known that most of mammalian mt-tRNAs exhibit non-canonical cloverleaf structure (17). For example, mt-tRNASer(UCN), which contains ms2i6A modification, exhibits relatively weak tertiary interactions between D-loop and T-loop when compared to the canonical cytosolic tRNA (18). The flexible structure might render mt-tRNASer(UCN) susceptive to denaturing condition during purification, which might cause the non-specific binding of mt-tRNASer(UCN) to oligo(dT) through A-rich region. Modifications in the residual mt-tRNAs in mRNA-enriched fraction will then give a strong background signal, which potentially leads to misinterpretation. Our study demonstrates that the residual RNA species in the purified mRNA fraction is not negligible in some cases, and these RNA species must be carefully verified by multiple approaches.
In this study, we generated a specific antibody against ms2i6A modification. This antibody showed a high specificity and was successfully applied for ELISA and immuno-blotting. Using this antibody, we presented data that strongly suggest that the ms2i6A modification exists exclusively in mt-tRNAs and not in other RNA species. Furthermore, we successfully applied the antibody to immunocytochemistry. We showed that ms2i6A-containing mt-tRNA was predominantly localized in mitochondria and was in the vicinity of TUFM, a component of the mitochondrial translation machinery. To our knowledge, this is the first report of the spatial distribution of ms2i6A-containing mt-tRNA in mammalian cells. The ms2i6A antibody developed in this study is a great tool for studying the cellular dynamics of mt-tRNAs. Furthermore, given that the ms2i6A modification is involved in the development of mitochondrial disease, the antibody might be useful for clinical diagnosis in the future.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Kazuto Nakata for the kind gift of HeLa ρo cells and B82 ρo cells. We thank Nobuko Maeda for her technical assistance in the animal studies.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Ministry of Education, Culture, Sports, Science and Technology of Japan Grant-in-aid for Scientific Research; Japan Agency for Medical Research and Development; Japan Science and Technology Agency; Takeda Science Foundation. Funding for open access charge: Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest statement. None declared.
REFERENCES
- 1. Agris P.F. Decoding the genome: a modified view. Nucleic Acids Res. 2004; 32:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Machnicka M.A., Milanowska K., Oglou O.O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M. et al. . MODOMICS: a database of RNA modification pathways - 2013 update. Nucleic Acids Res. 2013; 41:D262–D267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agris P.F., Vendeix F.A.P., Graham W.D.. tRNA’s wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2007; 366:1–13. [DOI] [PubMed] [Google Scholar]
- 4. Arragain S., Handelman S.K., Forouhar F., Wei F.-Y., Tomizawa K., Hunt J.F., Douki T., Fontecave M., Mulliez E., Atta M.. Identification of eukaryotic and prokaryotic methylthiotransferase for biosynthesis of 2-Methylthio- N6 -threonylcarbamoyladenosine in tRNA. J. Biol. Chem. 2010; 285:28425–28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei F.-Y., Suzuki T., Watanabe S., Kimura S., Kaitsuka T., Fujimura A., Matsui H., Atta M., Michiue H., Fontecave M. et al. . Deficit of tRNALys modification by Cdkal1 causes the development of type 2 diabetes in mice. J. Clin. Invest. 2011; 121:3598–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimas A.S., Lagou V., Barker A., Knowles J.W., Magi R., Hivert M.-F., Benazzo A., Rybin D., Jackson A.U., Stringham H.M. et al. . Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014; 63:2158–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie P., Wei F.-Y., Hirata S., Kaitsuka T., Suzuki T., Suzuki T., Tomizawa K.. Quantitative PCR measurement of tRNA 2-methylthio modification for assessing type 2 diabetes risk. Clin. Chem. 2013; 59:1604–1612. [DOI] [PubMed] [Google Scholar]
- 8. Zhou B., Wei F.-Y., Kanai N., Fujimura A., Kaitsuka T., Tomizawa K.. Identification of a splicing variant that regulates type 2 diabetes risk factor CDKAL1 level by a coding-independent mechanism in human. Hum. Mol. Genet. 2014; 23:4639–4650. [DOI] [PubMed] [Google Scholar]
- 9. Locke J.M., Wei F.-Y., Tomizawa K., Weedon M.N., Harries L.W.. A cautionary tale: the non-causal association between type 2 diabetes risk SNP, rs7756992, and levels of non-coding RNA, CDKAL1-v1. Diabetologia. 2015; 58:745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei F.-Y., Zhou B., Suzuki T., Miyata K., Ujihara Y., Horiguchi H., Takahashi N., Xie P., Michiue H., Fujimura A. et al. . Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 2015; 21:428–442. [DOI] [PubMed] [Google Scholar]
- 11. Reiter V., Matschkal D.M.S., Wagner M., Globisch D., Kneuttinger A.C., Müller M., Carell T.. The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res. 2012; 40:6235–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palazzo A.F., Lee E.S.. Non-coding RNA: what is functional and what is junk. Front. Genet. 2015; 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi N., Wei F.-Y., Watanabe S., Hirayama M., Ohuchi Y., Fujimura A., Kaitsuka T., Ishii I., Sawa T., Nakayama H. et al. . Reactive sulfur species regulate tRNA methylthiolation and contribute to insulin secretion. Nucleic Acids Res. 2017; 45:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okamaoto M., Ohsato T., Nakada K., Isobe K., Spelbrink J.N., Hayashi J.-I., Hamasaki N., Kang D.. Ditercalinium chloride, a pro-anticancer drug, intimately associates with mammalian mitochondrial DNA and inhibits its replication. Curr. Genet. 2003; 43:364–370. [DOI] [PubMed] [Google Scholar]
- 15. Zou X., Ji C., Jin F., Liu J., Wu M., Zheng H., Wang Y., Li X., Xu J., Gu S. et al. . Cloning, characterization and expression of CDK5RAP1_v3 and CDK5RAP1_v4, two novel splice variants of human CDK5RAP1. Genes Genet. Syst. 2004; 79:177–182. [DOI] [PubMed] [Google Scholar]
- 16. Telonis A.G., Loher P., Honda S., Jing Y., Palazzo J., Kirino Y., Rigoutsos I.. Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies. Oncotarget. 2015; 6:24797–24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki T.T., Nagao A., Suzuki T.T.. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 2011; 45:299–329. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe Y.-I., Kawai G., Yokogawa T., Hayashi N., Kumazawa Y., Ueda T., Nishikawa K., Hirao I., Miura K.-I., Watanabe K.. Higher-order structure of bovine mitochondrial tRNASerUGA: chemical modification and computer modeling. Nucleic Acids Res. 1994; 22:5378–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.