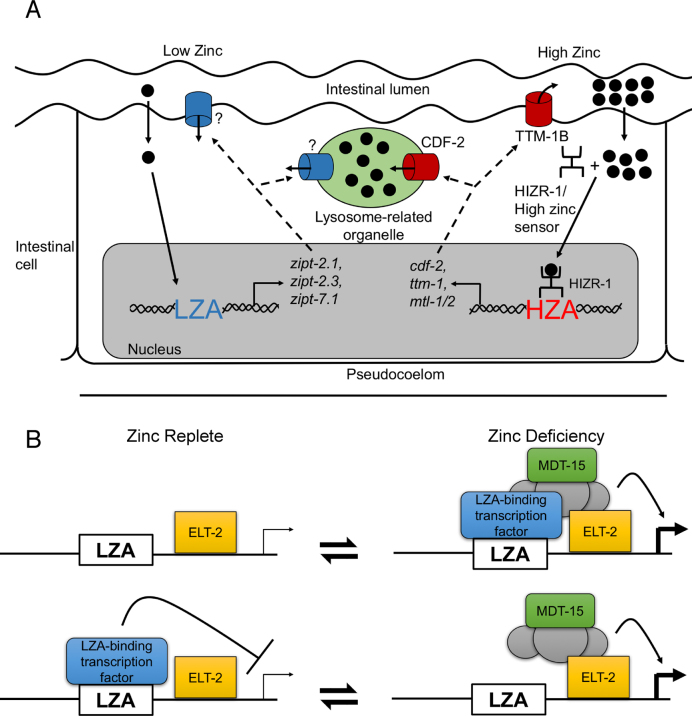
Figure 7.
Models of transcriptional regulation during zinc deficiency. (A) Diagram of an intestinal cell flanked by the intestinal lumen (above) and the pseudocoelom (below). Two dietary zinc-conditions are illustrated: deficiency (left) and excess (right). Zinc (black circles) enters the intestinal cell, and an undefined sensing mechanism causes activation of LZA element containing promoters. ZIPT proteins (blue) might localize to the plasma membrane or lysosome related organelles (LRO, green), but the subcellular localization has not been established. During zinc excess, the HIZR-1 nuclear receptor causes activation of HZA element containing promoters and the CDF-2 protein (red) localizes to the LRO where it functions to store zinc, and the TTM-1B protein (red) localizes to the plasma membrane where it functions to excrete zinc. (B) Two models of LZA element mediated transcriptional regulation. In the upper model, a proposed LZA element binding protein (blue box) is a transcriptional activator that interacts with the LZA element in response to zinc deficiency; this triggers assembly of a transcriptional activation complex including MDT-15 (green box). ELT-2 (yellow box) constitutively binds GATA elements and is permissive for intestinal cell expression. Stimulation is shown by an arrow and thick transcription start site. In the lower model, the LZA-element binding protein is a transcriptional repressor that interacts with the LZA element during zinc-replete conditions. Repression is shown by a bar and thin transcription start site; zinc deficiency abrogates the binding interaction, allowing assembly of a transcriptional activation complex. These two models are not mutually exclusive, since the LZA element binding protein might be converted from a repressor in zinc-replete conditions to an activator in zinc-deficient conditions.