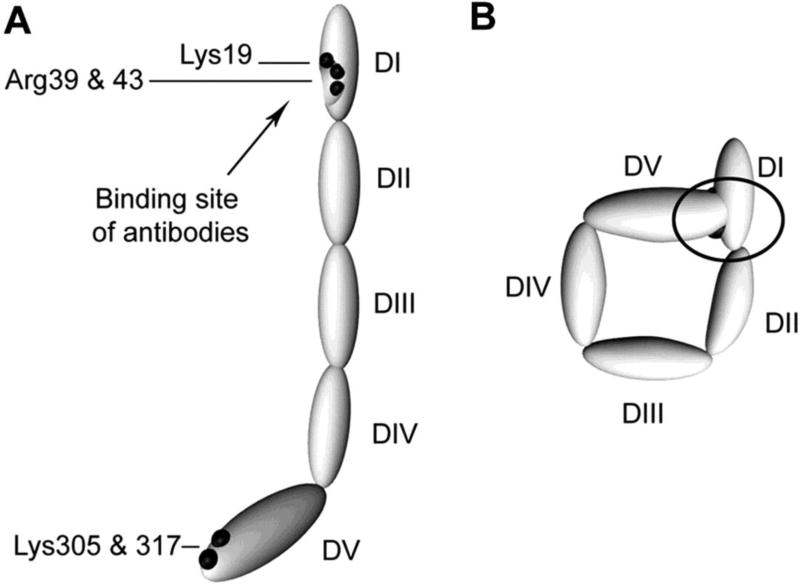
Figure 1. Proposed structures of the open and closed forms of β2GPI.
DI-DV represent the 5 domains of β2GPI. (A) β2GPI structure in the “open” form, as identified by its crystal structure. In this conformation, often referred to the “fish hook” conformation, an epitope containing Lys19, Arg39 and Arg43 that is recognized by anti-β2GPI domain 1 antibodies is exposed. β2GPI incubated at high pH adopts this conformation, and it is proposed that binding of anionic phospholipid results in similar conformational changes. (B) The “circular” form of β2GPI. This conformation is suggested by electron microscopy of circulating plasma β2GPI. In this conformation, the epitopes recognized by anti-β2GPI domain 1 antibodies are not available, which is thought to explain the fact that circulating immune complexes are not present in patients with antiphospholipid antibodies. This conformation is proposed to be maintained by interactions between domain 1 and domain 5. Adapted from Agar et al, Blood 116:1336, 2010 with permission.