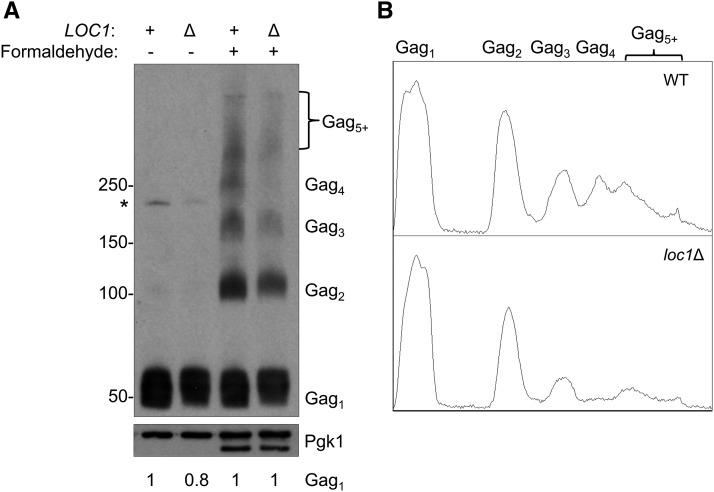
Figure 6.
Gag multimerization in wild type and loc1Δ strains. (A) Total protein from pBDG606-induced WT and loc1Δ strains were cross-linked by formaldehyde treatment in vivo. Protein extracts from untreated (− formaldehyde) and treated (+ formaldehyde) cells were separated on a 3–8% Tris-Acetate gel and probed with Gag antiserum (TY-tag). Equal amounts of protein separated on a 10% SDS-PAGE gel were probed with Pgk1 antiserum, which served as a loading control. Estimated protein sizes are shown in kilodaltons. Extracts from untreated cells contain monomeric Gag (Gag1) and the Gag-Pol-p199 precursor (*), while cross-linked cells produce a ladder of Gag multimers. The gel conditions allow sufficient separation of Gag1–4, while larger multimers (Gag5+) are not resolved. An additional faster-migrating Pgk1 signal appeared in formaldehyde-treated samples, which likely results from cross-linking. Numbers below the panel indicate relative changes in Gag1 level compared to wild type with and without formaldehyde treatment, as determined by quantification of protein bands by densitometry. Both Pgk1 bands in formaldehyde treated cells were used in the quantification. (B) Densitometry traces of cross-linked Gag from WT and loc1Δ. The pixel intensity for each Gag-multimer per lane is shown along the Y-axis.