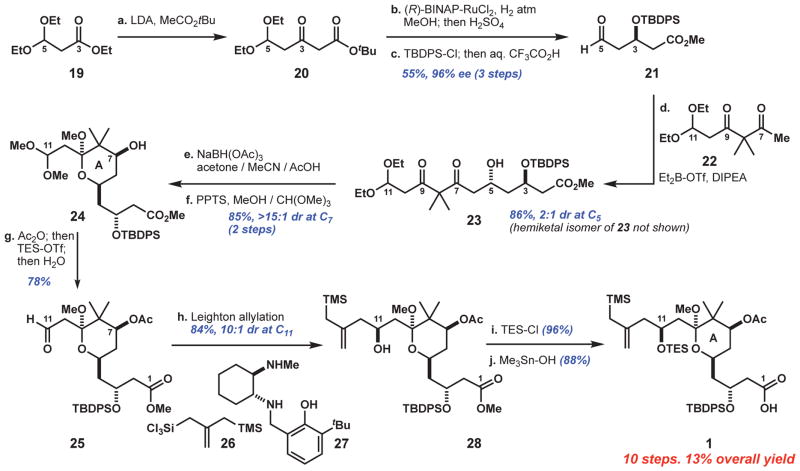
Fig. 3. Reaction sequence for the A-ring subunit, 1.
Reagents and conditions: a. n-BuLi (4 equiv), i-Pr2NH (4.1 equiv), THF, −78°C; then t-butyl acetate (4.05 equiv); then 19 (1 equiv), −78°C to room temperature. b. (R)-BINAP-RuCl2 (0.4 mol %), H2 (650 psi), MeOH, 45°C; then H2SO4 (7.5 mol%), 60°C. c. t-Butyldiphenylsilyl chloride (TBDPS-Cl) (1 equiv), imidazole (1.5 equiv), CH2Cl2; then 1:1 H2O:CF3CO2H, 0°C. d. Ketone 22 (2 equiv), diethylboron triflate (Et2B-OTf) (1.95 equiv), i-Pr2EtN (2 equiv), Et2O, −78°C; then aldehyde 21 (1 equiv), pentane. e. Sodium triacetoxyborohydride (7 equiv), 1:2:3 MeCN/acetone/AcOH, 0°C to room temperature. f. Pyridinium p-toluenesulfonate (PPTS) (1 equiv), 4:1 MeOH/CH(OMe)3. g. Acetic anhydride (Ac2O) (1.1 equiv), DMAP (10 mol %), 2,4,6-trimethylpyridine (9 equiv), CH2Cl2, −40°C; then triethylsilyl trifluoromethanesulfonate (TES-OTf) (5.5 equiv); then H2O, −40° to 0°C. h. Silane 26 (1.6 equiv), diaminophenol 27 (1.2 equiv), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (3.6 equiv), CH2Cl2, 0°C to room temperature; then aldehyde 25, −78°C; then tetrabutylammonium fluoride (TBAF) (1 equiv). i. Triethylsilyl chloride (TES-Cl) (1.5 equiv), imidazole (6 equiv), CH2Cl2, 0°C. j. Me3Sn-OH (3.5 equiv), toluene, 85°C; then Ac2O (5 equiv), DMAP (6 equiv), 0°C; then H2O, 0°C to room temperature.