Abstract
Women submitted to ART treatments represent a select subgroup of individuals. Several studies have described the relationship between TAI and pregnancy outcomes as a result of ART, with contradictory results. The purpose of this systematic review was to determine the association between TAI and the risk of miscarriage in pregnancies resulting from ART. MEDLINE via PubMed, LILACS and Embase were searched for studies published in peer-reviewed journals from 1999 to 2017. The studies were summarized using the fixed effects model and the Peto's method to calculate RR in order to flesh out the association between TAI and spontaneous abortion. Only four papers were included in this systematic review and meta-analysis. Thirty-one miscarriages were observed in 210 clinical pregnancies of women with antithyroid antibodies; and 158 miscarriages were seen in 1,371 pregnancies without antithyroid antibodies. The meta-analysis failed to find an association between TAI and higher risk of reproductive loss, RR=0.94 95% confidence interval: 0.71-1.24; p=0.879. In conclusion, the presence of antithyroid antibodies was not associated with increased reproductive loss in patients submitted to ART treatments. It is our opinion that the presence of antithyroid antibodies should be considered as a secondary biomarker of autoimmune disease, rather than an actual cause of miscarriage in patients undergoing ART. Due to the small amount of evidence on the matter, the determination of TAI before the initiation of ART should be limited to research contexts.
Keywords: ART, anitbodies, thyroid
INTRODUCTION
The percentage of women in the general population with thyroid autoimmunity (TAI), whether by thyroglobulin autoantibodies (anti-Tg) or anti-thyroid peroxidase antibodies (TPOAb), may be as high as 20% (Davies, 2016; Łukaszuk et al., 2015). In 1930, Stagnaro-Green et al. (1990) described the association between TAI and risk of spontaneous abortion. Since then, increased risk of fetal loss, perinatal mortality, and large for gestational age (LGA) newborns have been reported for euthyroid women with elevated concentrations of TPOAb (Bussen & Steck, 1995; Prummel & Wiersinga, 2004; Männistö et al. 2009). Other studies suggested that the presence of TAI in euthyroid women was associated with a 2-3 fold higher risk of miscarriage (Chen & Hu, 2011; Thangaratinam et al., 2011). The causality and pathophysiology of this association, or the adequate course of treatment, have not been completely elucidated.
Women submitted to assisted reproductive technology (ART) treatments represent a select subgroup of individuals. Several studies have described the relationship between TAI and pregnancy outcomes as a result of ART, with contradictory results (Abalovich et al., 2007; Stagnaro-Green & Glinoer, 2004). The purpose of this systematic review was to determine the association between TAI and the risk of miscarriage in pregnancies resulting from ART.
MATERIALS AND METHODS
Electronic search
MEDLINE via PubMed, LILACS and Embase were searched for papers written in English and Spanish published in peer-reviewed journals from 1999 to 2017. Search terms "antithyroid antibodies" "assisted reproductive techniques", and the combined MeSH terms "reproductive techniques, assisted OR fertilization in vitro OR sperm injections, intracytoplasmic" AND "thyroid gland" AND "autoantibodies" were used. Additional studies were found in the references of the retrieved papers.
Study selection
Studies looking into ART in women with ages ranging from 22 to 45 years were eligible for inclusion in the review. Secondary studies, studies without comparable groups, and studies performing Preimplantation Genetic Diagnosis were excluded.
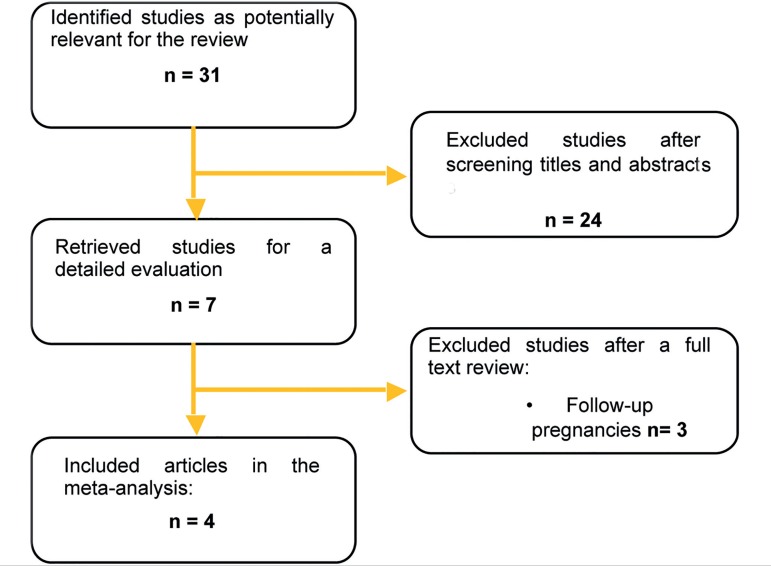
The papers were selected based on their titles and abstracts according to the inclusion criteria (Figure 1).
Figure 1.
Systematic review flowchart.
Selected outcomes
Papers comparing the pregnancy outcomes of individuals with and without TAI offered ART treatments were included, whereas studies not reporting pregnancy outcomes were not included (e.g. birth or miscarriage).
Data extraction
Two independent authors reviewed the titles and abstracts (PL and JES), and when applicable the full text was retrieved for further analysis. The independent authors assessed the papers for compliance with the inclusion criteria. Disagreements were resolved with the aid of a third author (CO). Data was extracted by one of the authors (PL) in a specially designed form that included references, study type, methods, results, and conclusions.
Methods of synthesis
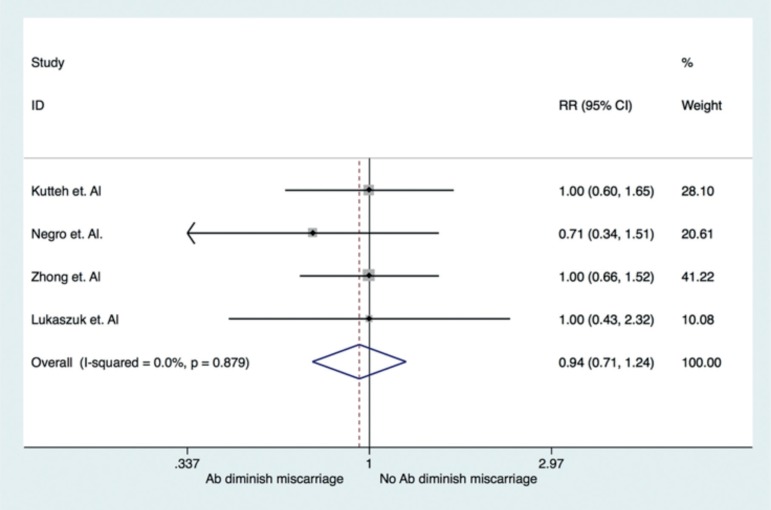
The studies were summarized using the fixed effects model and Peto´s method to calculate relative risk (RR) and 95% confidence intervals to further elicit the association between TAI and spontaneous abortion in women offered ART. Statistical analysis was performed on STATA 11.0 (STATA Corp, EEUU). The results were displayed in a forest plot (Figure 2).
Figure 2.
Forest plot: RR of miscarriage in TAI (+) patients undergoing ART.
Heterogeneity between studies was assessed with Higgins' I2 and Cochran's Q test. Heterogeneity was considered to be significant when p<0.01 and I2>30%.
RESULTS
The initial search retrieved 31 studies. No duplicates were found. After screening for titles and abstracts, 24 studies were excluded for not meeting the inclusion criteria. Seven remained for full text revisions, but three included ongoing pregnancies and were therefore excluded. Four papers met the inclusion criteria and were included in the systematic review and meta-analysis (Figure 2).
The four studies included were published between 1999 and 2015 and covered a total of 2,664 women offered ART. The main findings are summarized in Table 1. Two of the studies were carried out in Europe, one in Asia, and one in the United States. All were retrospective cohort studies.
Table 1.
Summary of included studies.
|
Author, year. Country |
Methods | TAI (+) | TAI (-) | Conclusions |
|---|---|---|---|---|
| Lukaszuk et al., 2015. China | Retrospective Cohort study of euthyroid
women, age 34-35 years, submitted to IVF/ICSI between April 2010 and
April 2012. Population was divided in 2 groups: patients with TPOAb+ (n=114) and TPOAb- (n=495) Measurement of TPOAb by ECLIA. Reference value: 0-34 IU/ml |
Clinical Pregnancy: n=50 Miscarriage: n=3 |
Clinical Pregnancy: n=235 Miscarriage: n=29 |
IVF patients undergoing ICSI with TPOAb+
vs TPOAb- did not present statistically significant differences in
fertilization, implantation, pregnancy, and live newborn
rates. The presence of TPOAb did not increase the risk of miscarriage (6% vs. 12.4%, p=0.29) |
| Zhong et al., 2012. Poland | Retrospective cohort study of patients
(mean age=32 years) submitted to IVF/ICSI between August 2009 and
August 2010. Population was divided in two groups: patients with TAI [TPOAb+ and/or anti-Tg+] (n=90) and without TAI (n=676) Measurement of TAI by CMIA: -TPOAb+ ≥561UI/ml -anti-Tg+] ≥4.11UI/ml |
Clinical Pregnancy: n=52 Miscarriage: n=14 |
Clinical Pregnancy: n=458 Miscarriage: n=54 |
Fertilization implantation and pregnancy rates after IVF-ET were significantly lower (64.3% vs. 74.6%, p<0.001; 17.8% vs. 27.1%, p<0.001; and 33.3% vs. 46.7%, p=0.002, respectively), whereas miscarriage rates were significantly higher (26.9% vs. 11.8%) in patients with TAI versus controls (without TAI). |
| Negro et al., 2007 Italy | Retrospective Cohort study of euthyroid
women aged 20-35 years, carried out between January 2000 and January
2005. A total of 416 patients were selected; 42 had TPOAb+ and 374 TPOAb-. Measurement method of TPOAb by RIA: -TPOAb+ ≥100 Ku/l |
Clinical Pregnancy: n=21 Miscarriage: n=5 |
Clinical Pregnancy: n=234 Miscarriage: n=27 |
In euthyroid patients, pregnancy and
delivery rates were not affected by the presence of TPOAb. In patients with TPOAb+, the subgroup of patients that did not achieve pregnancy had miscarriages had higher TSH levels, but within the normal range (2.8mUI/ml) vs. patients that delivered (TSH 1.06 mUI/ml, p=0.032) |
| Kutteh et al., 1999 USA | Retrospective cohort study of women aged
35±4 years offered IVF in 3 centers in the USA between April
1996 and April 1997. From a total of 873 patients with ART, 143 women had TAI [TPOAb+ and/or anti-Tg+]. Results were compared to a control group of 200 non-pregnant women of childbearing age with no record of reproductive problems Measurement of AIT with ELISA: -TPOAb+ ≥65 UI/ml. -anti-Tg+ ≥120 UI/ml. |
Clinical Pregnancy n=87 Miscarriage: n=9 |
Clinical Pregnancy: n=444 Miscarriage: n=48 |
The presence of TAI was similar between
patients offered ART and controls (16.4% vs. 14.5 %, OR:
1.16) No statistically significant differences were found in delivery (54.5% vs. 54.2%, p=1.00), biochemical miscarriage (3.5% vs. 4.7%, p=0.66), clinical miscarriage (6.3% vs. 6.6%, p=1.00), and pregnancy failure (35.7% vs. 34.5%, p=0.85) rates between patients with and without TAI. |
The groups were comparable for age in the selected papers. Only two studies mentioned the nutritional status of the patients with a mean body mass index of 22±4Kg/m2 (Łukaszuk et al., 2015; Zhong et al., 2012); two included only euthyroid women and two did not consider that condition, although they excluded individuals with other autoimmune diseases.
The methods used to determine the presence of TAI differed, as did their titrations: electrochemiluminescence immunoassay (Łukaszuk et al., 2015), chemiluminescent microparticle immunoassay (Zhong et al., 2012), radioimmunoassay (Negro et al., 2007), and enzyme-linked immunosorbent assay (Kutteh et al., 1999) (Table 1). In two studies the anti-Tg and anti-thyroid peroxidase antibodies levels were measured (Łukaszuk et al., 2015; Negro et al., 2007), while the other two only the level of anti-thyroid peroxidase antibodies was measured (Kutteh et al., 1999; Zhong et al., 2012).
Thirty-one miscarriages were observed in 210 clinical pregnancies of women with antithyroid antibodies; and 158 miscarriages were seen in 1,371 pregnancies without antithyroid antibodies. The meta-analysis failed to find an association between TAI and higher risk of reproductive loss, RR=0.94 95% confidence interval: 0.71-1.24; p=0.879 (Figure 2).
The heterogeneity between studies was not significant (p=0.879, I2=0.00%).
DISCUSSION
The purpose of this review was to determine whether pregnant individuals with TAI offered ART were at higher risk of having a miscarriage. After summarizing four studies including a total of 1,581 pregnancies after ART, no association was found between TAI and miscarriage. Most of the studies included in this meta-analysis showed no statistically significant differences in fertility rate, number of embryos available, implantation rate or clinical pregnancy rates between the groups. These findings were consistent with the results reported by Karacan et al. (2013), in which implantation rates, spontaneous abortion rates, and pregnancy rates did not differ significantly between the groups with TAI and without TAI.
The strength of this study resided in the large number of analyzed patients, a total of 2,664 individuals given ART treatments. A weakness of the study was the fact that levels of thyroglobulin autoantibodies (anti-Tg) were not measured in every included study, which have been reported to be around 5% in patients suffering from infertility (Unuane et al., 2013). Another limitation was that only two studies included euthyroid patients; the other two did not consider this trait in their inclusion criteria, but had presence of other autoimmune diseases as a criterion for exclusion. Levothyroxine was not prescribed to every patient included. Since TSH levels and prescription of levothyroxine seem to be relevant for IVF outcomes, Poppe et al. (2004) looked into the thyroid function of females submitted to IVF procedures and the possible associations with reproductive outcomes, and reported a modified pattern in thyroid function during the first period of pregnancy after comparing the groups with and without TAI.
Most of the patients did not receive levothyroxine during pregnancy. The meta-analysis performed by Velkeniers et al. (2013) and the study by Negro et al. (2005) found that thyroxine supplementation for women with subclinical hypothyroidism and/or thyroid autoimmunity might improve clinical pregnancy outcomes in patients offered ART, therefore such supplementation did not interfere with the outcomes described in our study.
The discrepancies between the results found in this study and in the papers written by Zhong et al. (2012) and Glinoer et al. (1991) may be explained by the mechanism of action of antithyroid antibodies; although this mechanism has not been completely described, it has been speculated that the anti-thyroid peroxidase antibodies can bind to the egg surface and/or embryo and interfere with fertilization and embryo development (Monteleone et al., 2011; Zhong et al., 2012). Our results, although contradictory at a first glance, seem to confirm this hypothesis: While TPOAb bound to the egg surface might prevent sperm cells from entering the egg during natural fertilization or IVF, it should not affect fertilization through intracytoplasmic sperm injection (ICSI), the method used in all patients in the included studies.
In conclusion, the presence of antithyroid antibodies was not associated with increased reproductive loss in patients submitted to ART treatments. It is our opinion that the presence of antithyroid antibodies should be considered as a secondary biomarker of autoimmune disease, rather than an actual cause of miscarriage in patients undergoing ART. Due to the small amount of evidence on the matter, the determination of TAI before the initiation of ART should be limited to research contexts.
REFERENCES
- Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S147–S147. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- Bussen S, Steck T. Thyroid autoantibodies in euthyroid non-pregnant women with recurrent spontaneous abortions. Hum Reprod. 1995;10:2938–2940. doi: 10.1093/oxfordjournals.humrep.a135823. [DOI] [PubMed] [Google Scholar]
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513–519. doi: 10.1111/j.1365-2265.2010.03974.x. [DOI] [PubMed] [Google Scholar]
- Davies T. UpToDatePost TW, editor. Pathogenesis of Hashimoto's thyroiditis (chronic autoimmune thyroiditis) UpToDate, Waltham, MA. [16/12/2016]. Available at: https://www.uptodate.com/contents/pathogenesis-of-hashimotos-thyroiditis-chronic-autoimmune-thyroiditis.
- Glinoer D, Soto MF, Bourdoux P, Lejeune B, Delange F, Lemone M, Kinthaert J, Robijn C, Grun JP, de Nayer P, editors. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421–427. doi: 10.1210/jcem-73-2-421. [DOI] [PubMed] [Google Scholar]
- Karacan M, Alwaeely F, Cebi Z, Berberoglugil M, Batukan M, Ulug M, Arvas A, Camlıbel T, editors. Effect of antithyroid antibodies on ICSI outcome in antiphospholipid antibody-negative euthyroid women. Reprod Biomed Online. 2013;27:376–380. doi: 10.1016/j.rbmo.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Kutteh WH, Schoolcraft WB, Scott RT Jr, editors. Antithyroid antibodies do not affect pregnancy outcome in women undergoing assisted reproduction. Hum Reprod. 1999;14:2886–2890. doi: 10.1093/humrep/14.11.2886. [DOI] [PubMed] [Google Scholar]
- Łukaszuk K, Kunicki M, Kulwikowska P, Liss J, Pastuszek E, Jaszczołt M, Męczekalski B, Skowroński K, editors. The impact of the presence of antithyroid antibodies on pregnancy outcome following intracytoplasmatic sperm injection-ICSI and embryo transfer in women with normal thyreotropine levels. J Endocrinol Invest. 2015;38:1335–1343. doi: 10.1007/s40618-015-0377-5. [DOI] [PubMed] [Google Scholar]
- Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, Bloigu A, Järvelin MR, Suvanto-Luukkonen E, editors. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab. 2009;94:772–779. doi: 10.1210/jc.2008-1520. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Parrini D, Faviana P, Carletti E, Casarosa E, Uccelli A, Cela V, Genazzani AR, Artini PG, editors. Female infertility related to thyroid autoimmunity: the ovarian follicle hypothesis. Am J Reprod Immunol. 2011;66:108–114. doi: 10.1111/j.1600-0897.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, Locorotondo G, Caroli P, Pezzarossa A, Dazzi D, Hassan H, editors. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod. 2005;20:1529–1533. doi: 10.1093/humrep/deh843. [DOI] [PubMed] [Google Scholar]
- Negro R, Formoso G, Coppola L, Presicce G, Mangieri T, Pezzarossa A, Dazzi D, editors. Euthyroid women with autoimmune disease undergoing assisted reproduction technologies: the role of autoimmunity and thyroid function. J Endocrinol Invest. 2007;30:3–8. doi: 10.1007/BF03347388. [DOI] [PubMed] [Google Scholar]
- Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Devroey P, van Steirteghem A, Haentjens P, Velkeniers B, editors. Impact of ovarian hyperstimulation on thyroid function in women with and without thyroid autoimmunity. J Clin Endocrinol Metab. 2004;89:3808–3812. doi: 10.1210/jc.2004-0105. [DOI] [PubMed] [Google Scholar]
- Prummel MF, Wiersinga WM, editors. Thyroid autoimmunity and miscarriage. Eur J Endocrinol. 2004;150:751–755. doi: 10.1530/eje.0.1500751. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Alvarez-Marfany M, Davies TF, editors. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990;264:1422–1425. doi: 10.1001/jama.1990.03450110068029. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A, Glinoer D, editors. Thyroid autoimmunity and the risk of miscarriage. Best Pract Res Clin Endocrinol Metab. 2004;18:167–181. doi: 10.1016/j.beem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A, editors. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011;342:d2616. doi: 10.1136/bmj.d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unuane D, Velkeniers B, Anckaert E, Schiettecatte J, Tournaye H, Haentjens P, Poppe K, editors. Thyroglobulin autoantibodies: is there any added value in the detection of thyroid autoimmunity in women consulting for fertility treatment? Thyroid. 2013;23:1022–1028. doi: 10.1089/thy.2012.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P, editors. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta-analysis of RCTs. Hum Reprod Update. 2013;19:251–258. doi: 10.1093/humupd/dms052. [DOI] [PubMed] [Google Scholar]
- Zhong YP, Ying Y, Wu HT, Zhou CQ, Xu YW, Wang Q, Li J, Shen XT, Li J, editors. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci. 2012;9:121–125. doi: 10.7150/ijms.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]