Figure 2.
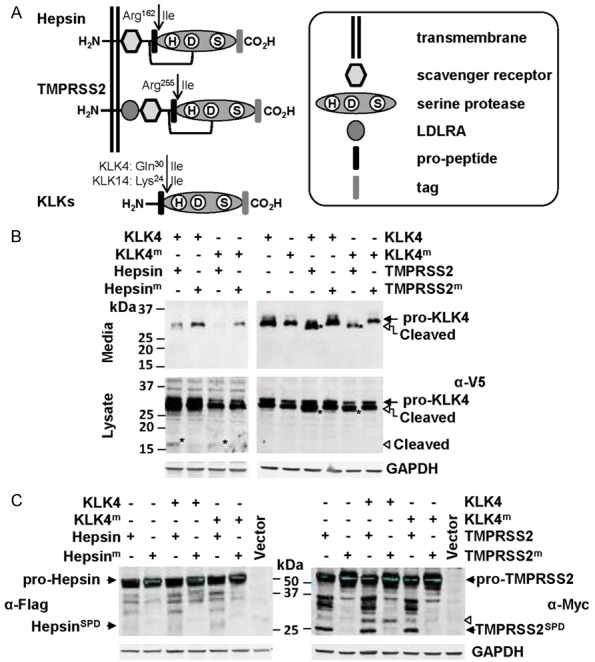
The secreted serine protease KLK4 is cleaved, and potentially transiently activated, at the cell surface by the plasma membrane anchored serine proteases hepsin and TMPRSS2. A. Schematic representation of the structure of hepsin, TMPRSS2 and KLKs. B. Anti-V5 Western blot analysis for KLK4-V5 of conditioned media and lysates from cells co-transfected with wild type or active-site mutated KLK4-V5 (KLK4; KLK4m) and wild type or active-site mutated hepsin-Flag (hepsin; hepsinm) or wild type or active-site mutated TMPRSS2-Myc (TMPRSS2; TMPRSS2m). Arrowhead indicates a 17 kDa KLK4 degradation product. C. Anti-Flag and anti-Myc Western blot analysis for hepsin-Flag and TMPRSS2-Myc of lysates from cells co-expressing KLK4 or KLK4m with hepsin or hepsinm, or KLK4 or KLK4m with TMPRSS2 or TMPRSS2m. Arrowhead indicates a KLK4 generated TMPRSS2 fragment of ~30 kDa.
