Figure 4.
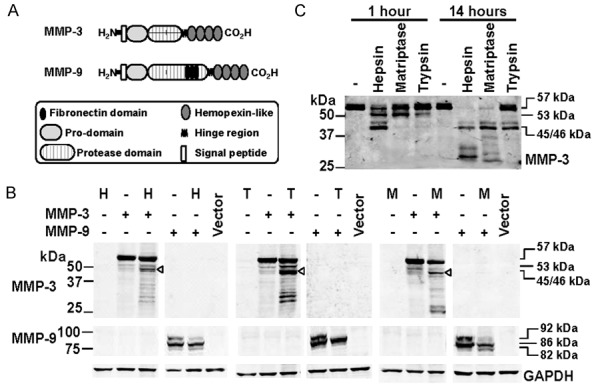
Examination of proteolytic processing of MMP-3 and MMP-9 by hepsin and TMPRSS2. A. Schematic representation of the structure of MMP-3 and MMP-9. B. Anti-MMP-3 (top), -MMP-9 (middle) and -GAPDH (bottom) Western blot analyses of lysates from cells co-transfected with hepsin (H), TMPRSS2 (T) or matriptase (M) and MMP-3 or MM-P-9. ProMMP-3, 57 kDa; intermediate MMP-3, 53 kDa; activated MMP-3, 45/46 kDa. Pro-MMP-9, 92 kDa; intermediate MMP-9, 86 kDa; activated MMP-9, 82 kDa. C. Anti-MMP-3 Western blot analysis of conditioned media from cells transiently expressing MMP-3 or MMP-9 that had been incubated for 1 or 14 h with recombinant hepsin (50 nM), recombinant matriptase (50 nM) or bovine trypsin (10 nM). ProMMP-3, at 57 kDa, intermediate MMP-3 at 53 kDa, and activated MMP-3 at 45/46 kDa are indicated.
