Abstract
Objective: This study aimed to investigate whether tumor-associated macrophages (TAMs) and esophageal squamous cell carcinoma (ESCC) cells could synergistically influence the generation of lymphatic vessels via the VEGF-C/VEGFR-3 signaling pathway and to address its mechanism. Methods: M2 macrophages were sorted with immunomagnetic beads and induced in vitro. VEGF-C siRNA plasmids were constructed and transfected into M2 macrophages and the ESCC cell line KYSE150. Different conditioned culture media before and after transfection were collected and classified into different groups for culturing ESCC-associated lymphatic endothelial cells (ESCC-LECs). Using the CCK-8 assay, Transwell cell migration assay and Matrigel three-dimensional culture, the proliferation, migration and ring forming abilities of ESCC-LECs before and after transfection were compared, respectively. With ELISA, western blot and q(RT)-PCR, VEGF-C concentrations in conditioned culture media and the protein and mRNA expression levels of VEGFR-3 in LECs before and after transfection were compared, respectively. Results: Before transfection, ESCC-LECs in the group with mixed culture medium had stronger proliferation, migration and ring forming abilities than the other groups. The VEGF-C concentration and VEGFR-3 protein and mRNA expression levels were higher in the mixed culture medium group than in the other groups. After transfection, all indices were the lowest in the mixed culture medium group. Conclusions: M2 macrophages can enhance the proliferation, migration and ring forming abilities of ESCC-LECs. ESCC cells and M2 macrophages have synergistic effects on the proliferation, migration and ring forming abilities of ESCC-LECs. VEGF-C siRNA can inhibit the proliferation, migration and ring forming abilities of ESCC-LECs by silencing the expression of VEGF-C and its receptor VEGFR-3 in KYSE150 cells and M2 macrophages.
Keywords: TAMs, VEGF-C, esophageal squamous cell carcinoma-associated LECs, RNA interference, synergistic influence
Introduction
Esophageal cancer is a common type of gastrointestinal malignancy, ranking eighth among malignant carcinomas worldwide. Its five-year survival rate is only 15-25% [1,2]. Lymphatic metastasis is the most common and important metastatic route and may occur in the early phase of disease. Patient therapy, disease recurrence and long-term survival will be profoundly influenced by investigating the mechanism of lymphatic metastasis in esophageal cancer and by taking effective measures to inhibit the generation of tumor lymphatic vessels.
Lymphatic vessel invasion and metastasis of tumor cells are mainly presented as invasion into the existing peri-tumor lymphatic vessels and hyperplasia of lymphatic endothelial cells (LECs) [3,4]. Vascular endothelial growth factor C (VEGF-C) is the major cytokine that promotes the generation of lymphatic vessels [5,6]. VEGF-C binding to VEGFR-3 on LECs can promote the proliferation and differentiation of LECs [7]. A great deal of research has proven that tumor cells can secrete VEGF-C and that tumor-associated macrophages (TAMs) may also participate in the generation of lymphatic vessels and in the regional metastasis of lymph nodes through the VEGF-C/VEGFR-3 signal transduction pathway [8-10].
It has been determined that proliferation and differentiation abilities are decreased significantly in tumor cells transfected with VEGF-C siRNA in vitro, but in vivo experiments have revealed that the generation of lymphatic vessels in implanted tumor and peri-tumor tissues of nude mice is not reduced significantly. It was conjectured that inhibition of VEGF-C expression in only tumor cells was not sufficient to prevent the generation of lymphatic vessels. Thus, it was suggested that by inhibiting both the production of VEGF-C in M2 macrophages and VEGF-C expression in esophageal squamous cell carcinoma (ESCC) cells, the proliferation of ESCC-associated LECs (ESCC-LECs) and the generation of lymphatic vessels might be effectively prevented so that the lymphatic metastasis of esophageal cancer and other malignancies might be blocked. The present study aimed to provide a theoretical basis for blocking the invasion and metastasis of ESCC and other metastatic malignancies and for improving the therapeutic side effects and prognosis of patients with tumors.
Materials and methods
Reagents and instruments
A Miltenyi Automatic Magnetic Activated Cell-sorting System and Particle Analyzing System (PAS) were utilized in our experiments. CD14 immunomagnetic beads were purchased from Miltenyi Biotech Co., Germany.
Cells
Human esophageal squamous cell carcinoma KYSE150 cells were purchased from the Cell Bank of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Human esophageal squamous cell carcinoma-associated LECs were purchased from Shanghai Bioleaf Biotech Co.
Sorting and primary culture of M2 macrophages
Peripheral blood mononuclear cells (PBMCs) were collected from the peripheral blood of healthy individuals by density gradient centrifugation. The positive cells sorted with the CD14 immunomagnetic beads were considered mononuclear cells. Macrophage colony-stimulating factor (M-CSF, 10 ng/ml) and IL-4 (20 ng/ml) were sequentially added to the PBMC culture medium for 6 days and 1 day, respectively. Then, PAS examination was performed.
Cell transfection
Three segments of siRNA were designed and synthesized (Table 1, Shanghai GenePharma Co., Ltd.). Transfection buffer was prepared by thoroughly mixing the diluted liposome RNAiMAX and siRNA with the optimal concentrations of siRNA at 8 nmol/L, 12 nmol/L or 20 nmol/L. Twenty-four hours later, the transfection efficiency was observed with PAS and a fluorescence, inverted microscope to choose the optimal concentration for siRNA transfection. The effects of gene silencing were detected by q(RT)-PCR to select the optimal siRNA sequence.
Table 1.
VEGF-CSI RNA sequence
| siRNA | Sequence | |
|---|---|---|
| VEGF-C siRNA-1 (S1) | Sense | 5’-CUUAUGCAAGCAAAGAUCUTT-3’ |
| Antisense | 5’-AGAUCUUUGCUUGCAUAAGCC-3’ | |
| VEGF-C siRNA-2 (S2) | Sense | 5’-AAUUACAUGUGGAAUAAUCTT-3’ |
| Antisense | 5’-GAUUAGUCCACAUGUAAUUGG-3’ | |
| VEGF-C siRNA-3 (S3) | Sense | 5’-CGAUGCAUGUCUAAACUGGTT-3’ |
| Antisense | 5’-CCAGUUUAGACAUGCAUCGGC-3’ | |
| Negative control siRNA (NC) | Sense | 5’-GUCAAUGGUCGUGUAGAGUTT-3’ |
| Antisense | 5’-ACUCUACACGACCAUUGACTT-3’ | |
Collection and classification of conditioned culture medium
Cell groups before transfection were defined as follows: A: M2 macrophage and KYSE150 cell mixed culture medium; B: M2 macrophage-conditioned culture medium; C: KYSE150 cell-conditioned culture medium; and D: RPMI 1640 whole culture medium.
Cell groups after transfection were defined as follows: Z1: post-transfection mixed culture medium; Z2: post-transfection M2 macrophage-conditioned culture medium; Z3: post-transfection KYSE150 cell-conditioned culture medium; and Z4: RPMI 1640 whole culture medium.
Analysis of cell proliferation
Cells from the above groups were seeded in a 96-well plate. Each group was assigned 5 repetitive wells and cultured for 6, 12, 24, 36 and 48 h. After 100 μl of CCK-8 buffer was added into each well, the plate was incubated in an incubator for 2 h and examined by a microplate reader. ESCC-LEC proliferation curves were determined.
Determination of cell migration ability
The ESCC-LEC suspension (100 μl) was added to the upper chamber of a Transwell chamber, and 600 μl of each conditioned culture medium, which had already been prepared, was added to the lower chamber with 3 repetitive wells for each group. Twenty-four hours later, the cells on the lower surface of the polycarbonate film were observed and counted under an inverted microscope.
Ring forming ability of the cells
Cells from each group (5.0×105/ml) were inoculated into the culture plate covered with Matrigel with 3 repetitive wells for each group. Twenty-four hours later, the array of tubal structures and the integrity of these structures in each group were observed under an inverted microscope.
Determination of VEGFR-3 mRNA expression by q(RT)-PCR
Total RNA was extracted, and its concentration was determined. RNA was reverse-transcribed into cDNA and subjected to amplification. Preparation of the PCR amplification system is presented in Table 2. PCR amplification conditions were as follows: preheating at 95°C for 30 s, denaturation at 95°C for 3 s, and 60°C for 30 s. A melting curve was generated, and the samples were stored at 4°C. The standard curve was delineated with 7500 Software v2.3, and the CT values were analyzed. The primer sequences for the VEGFR-3 gene are as follows: F: 5’-GAGAGAGAGAAGGCAGCATAC-3’; R: 5’-GTGCTTCAGTGGTCACACTCC-3’. The primer sequences for β-actin are as follows: F: 5’-AGCGAGCATCCCCCAAAGTT-3’; R: 5’-GGGCACGAAGGCTCATCATT-3. The primers were synthesized by Shanghai Sangon Biotech Co. Ltd.
Table 2.
Preparation of PCR amplification reaction system
| Reagent name | Usage amount (μl) |
|---|---|
| SYBR Premix Ex Taq (Tli RNaseH Plus) (2×) | 10 |
| PCR Forward Primer (10 μM) | 0.8 |
| PCR Reverse Primer (10 μM) | 0.8 |
| ROX Reference Dye II (50×) | 0.4 |
| cDNA solution | 2 |
| dH2O (Sterilized distilled water) | 6 |
| Total | 20 |
Determination of VEGFR-3 protein expression by Western blotting
Cells were lysed, and total protein was extracted. With β-actin as the internal standard, the integral optical density ratios of VEGFR-3 protein and β-actin in each group of cells were measured routinely.
Statistical analysis
The data are expressed as the mean ± SD and were analyzed by SPSS 17.0. Means of multiple groups were compared by one-way analysis of variance (ANOVA), and variable transformation was applied to adjust for heterogeneous variance. The significance level was set as α=0.05.
Results
Cell morphological observations
Mononuclear cells were partly adherent, round, and relatively small with complete membranes. After sequential induction with M-CSF and IL-4, many typical, adherent cells could be seen with substantially greater volumes. The cells were stretched, and some of them were fusiform with dendrite-like structures (Figure 1A-D).
Figure 1.
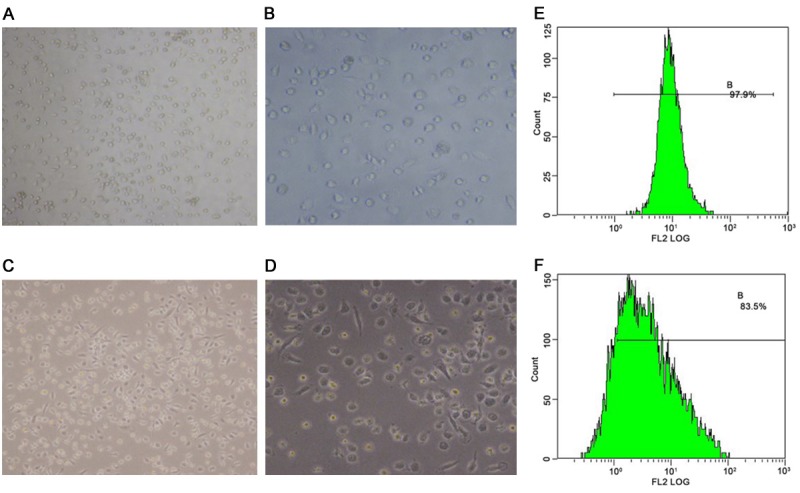
Cell morphological observation and phenotype identification by PAS. (A) Mononuclear cells (×100 magnification), (B) mononuclear cells (×200 magnification), (C) M2 macrophages (×100 magnification), (D) M2 macrophages (×200 magnification), (E) CD14+ mononuclear cells, and (F) percentage of M2 macrophages (CD163+).
Phenotype identification by PAS
After the cells were sorted with immunomagnetic beads, the positive rate of CD14 cells was 94.63±3.46% (Figure 1E). After induction into M2 macrophages with sequential M-CSF and IL-4 treatment, the positive rate of M2 macrophages was 81.3±2.66% (Figure 1F).
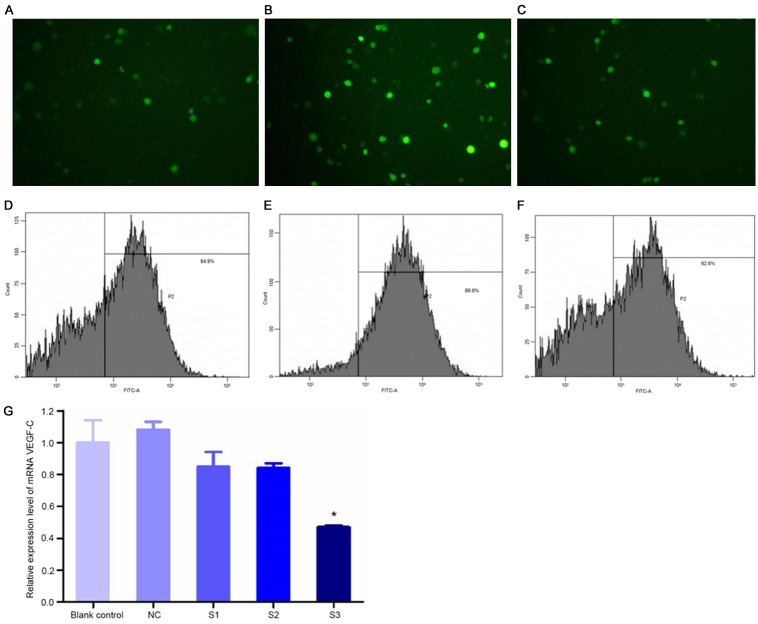
Efficiency of transfection
Cells transfected with the three concentrations of siRNA all produced green fluorescence, and the fluorescence was strongest at the concentration of 12 nmol/L (Figure 2A-C). The efficiency of transfection at concentrations of 8 nmol/L, 12 nmol/L and 20 nmol/L was 62.5±3.03%, 88.97±3.62% and 60.00±7.44%, respectively (Figure 2D-F).
Figure 2.
Efficiency of VEGF-C siRNA plasmid transfection (A-F) and relative mRNA expression levels of VEGF-C in cells transfected with different plasmids (G). (A-C) ×200 magnification, (D) 8 nmol/L siRNA, (E) 12 nmol/L siRNA, and (F) 20 nmol/L siRNA. *P<0.05, compared with the blank control. S1, S2 and S3 are three different sequences of siRNA. NC is the group transfected with an unrelated siRNA sequence.
Effects of gene silencing
Compared with the control group, the mRNA expression level of the VEGF-C gene in cells transfected with the S3 plasmid was reduced below 50%, indicating that the inhibitory effect of the S3 plasmid was superior to that of the plasmids in other groups (Figure 2G).
Analysis of cell proliferation
Before transfection, compared with group D, LEC proliferation abilities in groups A, B and C were enhanced (P<0.05), and the proliferation ability was strongest in group A, which was higher than in group B and group C (P<0.05, Figure 3A and 3B). After transfection, compared with the Z4 group, cell proliferation abilities in the Z1, Z2 and Z3 group were significantly reduced (P<0.05), and the proliferation abilities in the Z2 and Z3 group were higher than in Z1 group (P<0.05, Figure 3C and 3D).
Figure 3.
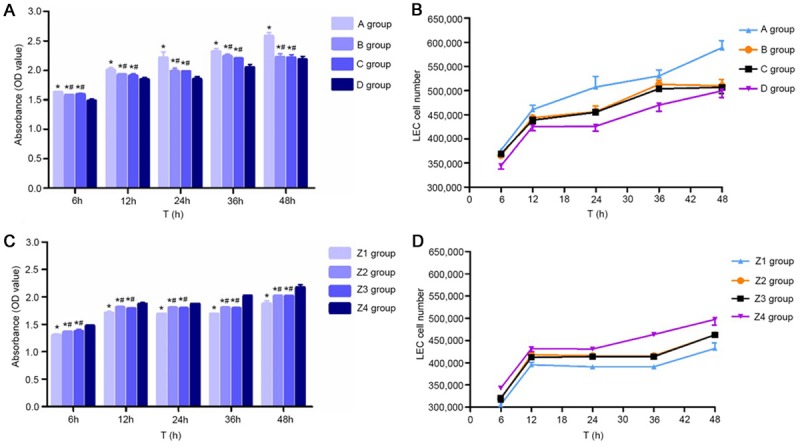
Proliferation of ESCC-LECs in different groups before and after transfection. A: Proliferation (OD values) of ESCC-LECs in different groups. *P<0.05, compared with group D. #P<0.05, compared with group A. B: Proliferation curves of ESCC-LECs (n=3). (A) Mixed culture medium group, (B) M2 macrophage group, (C) KYSE150 group, and (D) control group. C: After transfection, the proliferation (OD values) of ESCC-LECs cultured in conditioned culture medium. *P<0.05, compared with group Z4; #P<0.05, compared with group Z1. D: After transfection, the proliferation curves of ESCC-LECs (n=3). (Z1) Post-transfection mixed culture medium group, (Z2) post-transfection M2 macrophage group, (Z3) post-transfection KYSE150 group, and (Z4) control group.
Cell migration
By observation under a fluorescence inverted microscope, cells were counted and averaged in five fields: upper, lower, left, right and central. Before transfection, compared with group D, the number of migrating cells in group A, group B and group C increased (P<0.05), and the number of migrating cells in group B and C was less than in group A (P<0.05, Figure 4A). After transfection, compared with group Z4, the number of migrating cells in group Z1, group Z2 and group Z3 increased (P<0.05), and the number of migrating cells in group Z2 and group Z3 was less than in group A (P<0.05, Figure 4B).
Figure 4.
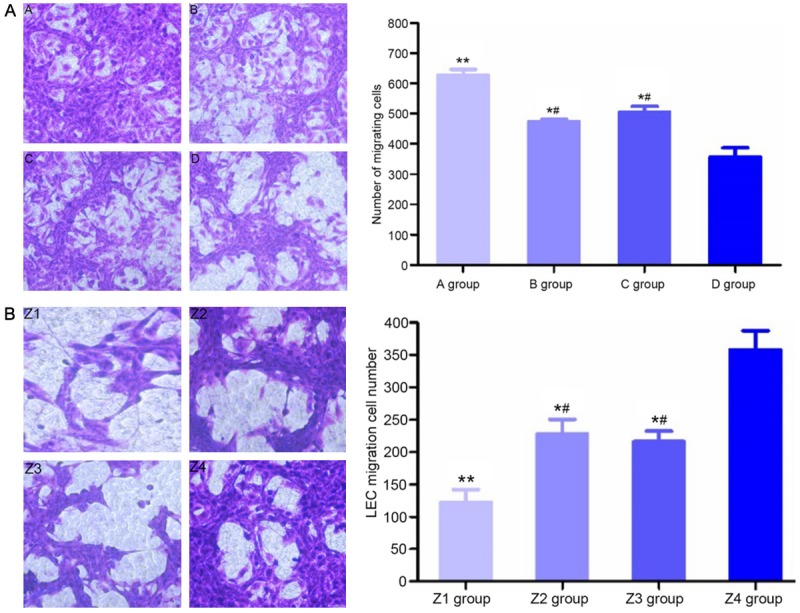
Migrating cell counts and morphology of different groups of ESCC-LECs before (A) and after transfection (B) (×200 magnification, the left image). **P<0.01, compared with group D; *P<0.05, compared with group D; #P<0.05, compared with group A. (A) Mixed culture medium group, (B) M2 macrophage group, (C) KYSE150 group, (D) control group, (Z1) post-transfection mixed culture medium group, (Z2) post-transfection M2 macrophage group, (Z3) post-transfection KYSE150 group, and (Z4) control group.
Ring forming ability of cells
With Matrigel supporting the lymphatic vessel-like structures, the structures were observed under an inverted microscope. Before transfection, the number of cell rings in group A, group B and group C was greater than in the control group (P<0.05), with the most rings observed in group A (P<0.05, Figure 5A). After transfection, the number of cell rings in group Z1, group Z2 and group Z3 decreased (P<0.05), with the fewest rings observed in group Z1 (P<0.05, Figure 5B).
Figure 5.
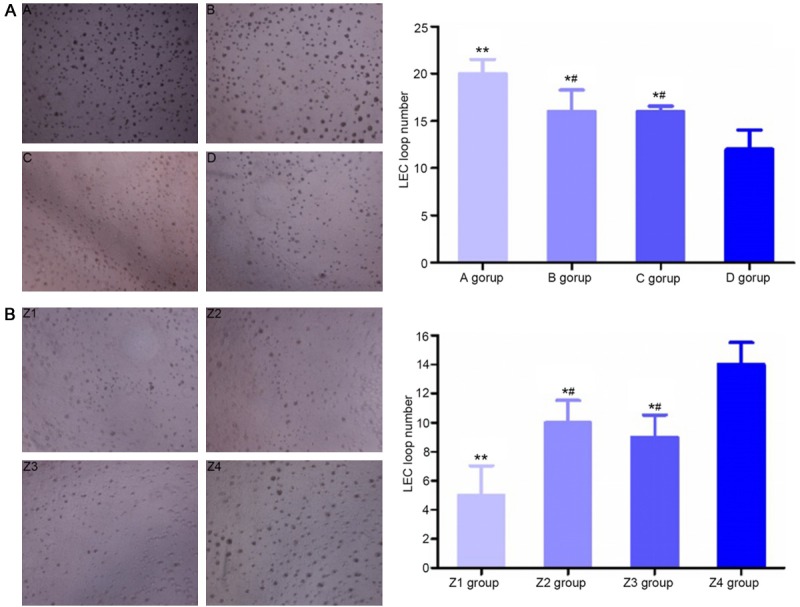
The number of cell rings in different groups of ESCC-LECs (the right image) and cell morphology (the left image, ×200 magnification) before and after transfection. A: **P<0.01, compared with group D; *P<0.05, compared with group D; #P<0.05, compared with group A. (A) Mixed culture medium group, (B) M2 macrophage group, (C) KYSE150 group, and (D) control group. B: **P<0.01, compared with group Z4; *P<0.05, compared with group Z4; #P<0.05, compared with group Z1. (Z1) Post-transfection mixed culture medium group, (Z2) post-transfection M2 macrophage group, (Z3) post-transfection KYSE150 group, and (Z4) control group.
Determination of the VEGF-C concentration
Before transfection, the concentration of VEGF-C in the conditioned culture media of group A, group B and group C was higher than in that of the control group (P<0.05), and the concentrations of VEGF-C in the conditioned culture media of group B and group C were lower than in that of group A (P<0.05, Figure 6A). After transfection, compared with the control group, VEGF-C concentrations in the conditioned culture media of group Z1, group Z2 and group Z3 were all decreased (P<0.05), with higher concentrations in the conditioned culture media of group Z2 and group Z3 than in that of group Z1 (P<0.05, Figure 6B).
Figure 6.
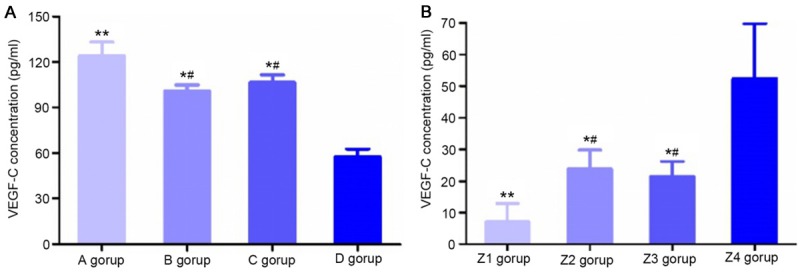
Determination of VEGF-C concentration. A: VEGF-C concentrations (pg/ml) in conditioned culture medium of different groups. **P<0.01, compared with group D; *P<0.05, compared with group D; #P<0.05, compared with group A. (A) Mixed culture medium group, (B) M2 macrophage group, (C) KYSE150 group, and (D) control group. B: After transfection, VEGF-C concentrations (pg/ml) in conditioned culture media of different groups. **P<0.01, compared with group Z4; *P<0.05, compared with group Z4; #P<0.05, compared with group Z1. (Z1) Post-transfection mixed culture medium group, (Z2) post-transfection M2 macrophage group, (Z3) post-transfection KYSE150 group, and (Z4) control group.
VEGFR-3 mRNA expression
Before transfection, VEGFR-3 mRNA expression levels in group A, group B and group C were all higher than in group D. In group A, the expression level increased by 4.62-fold (P<0.01), and in group B and group C, the levels increased by 1.95- and 2.08-fold, respectively (P<0.05). The mRNA expression levels in group B and group C were lower than in group A (P<0.05, Figure 7A). After transfection, compared with group Z4, VEGFR-3 mRNA expression levels in group Z1, group Z2 and group Z3 were decreased; VEGFR-3 mRNA expression levels significantly decreased by 77% in group Z1 (P<0.01) and by 44% and 45% in group Z2 and group Z3, respectively (P<0.05). The VEGFR-3 mRNA expression levels in group Z2 and group Z3 were higher than in group Z1 (P<0.05, Figure 7B).
Figure 7.
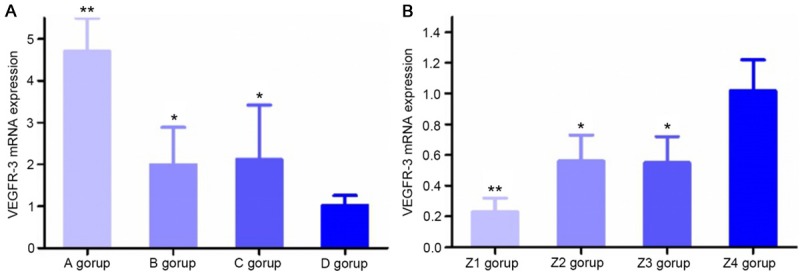
mRNA expression of VEGFR-3. A: VEGFR-3 mRNA expression in cells of different groups. **P<0.01, compared with group D; *P<0.05, compared with group D. (A) Mixed culture medium group, (B) M2 macrophage group, (C) KYSE150 group, and (D) control group. B: After transfection, VEGFR-3 mRNA expression in LECs of different groups. **P<0.01, compared with group Z4; *P<0.05, compared with group Z4. (Z1) Post-transfection mixed culture medium group, (Z2) post-transfection M2 macrophage group, (Z3) post-transfection KYSE150 group, and (Z4) control group.
VEGFR-3 protein expression
Before transfection, VEGFR-3 protein expression levels in group A, group B and group C were all higher compared to that in group D (P<0.05), and the levels in group B and group C were lower than that in group A (P<0.05, Figure 8A). After transfection, compared with that in group Z4, VEGFR-3 protein expression levels were decreased in group Z1, group Z2 and group Z3 (P<0.05), and the levels in group Z2 and group Z3 were higher than that in group Z1 (P<0.05, Figure 8B).
Figure 8.
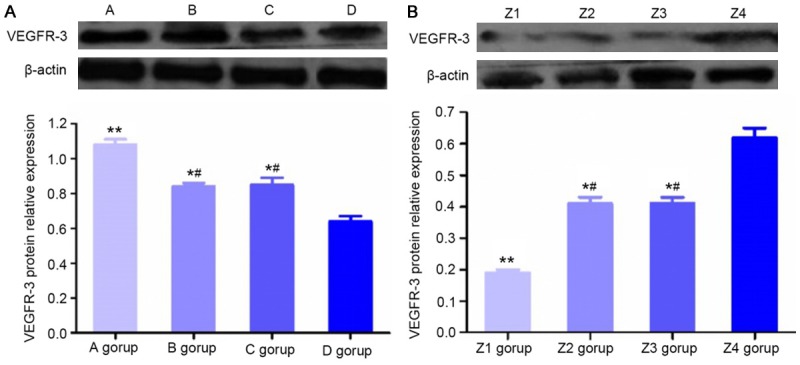
Protein expression of VEGFR-3. A: Relative protein expression levels of VEGFR-3 in cells of different groups. **P<0.01, compared with group D; *P<0.05, compared with group D; #P<0.05, compared with group A. (A) Mixed culture medium group, (B) M2 macrophage group, (C) KYSE150 group, and (D) control group. B: After transfection, VEGFR-3 protein expression levels in LECs of different groups. **P<0.01, compared with group Z4; *P<0.05, compared with group Z4; #P<0.05, compared with group Z1. (Z1) Post-transfection mixed culture medium group, (Z2) post-transfection M2 macrophage group, (Z3) post-transfection KYSE150 group, and (Z4) control group.
Discussion
The generation of tumor lymphatic vessels is a complicated, continuous process. In animal experiments, Kozaki K. et al. found that overexpression of VEGF-C in tumor cells was positively correlated with the generation of lymphatic vessels and lymphatic metastasis, while inhibiting the VEGF-C signaling pathway could produce inhibitory effects on tumor metastasis to some extent [11]. Current research suggests that the VEGFR-3-mediated signal transduction pathway plays a major role in regulating lymphatic vessel generation. However, studies by Witte D. and Arinaga reported that the expression level of VEGF-C/VEGFR-3 had no direct association with lymphatic metastasis in lung cancer or colorectal cancer [12,13]. This discrepancy may be attributed to the difference in anatomical regions where the tumors originated or in the experimental protocols.
Jetlsch et al. found that VEGF-C was overexpressed in transgenic mice that displayed hyperplasia of skin lymphatic vessels, which might result from VEGF-C binding and the activation of VEGFR-3 in the lymphatic endothelium [14,15]. The present study suggests that ESCC cells can secrete lymphatic vessel growth factor VEGF-C, which mainly functions through binding to VEGFR-3 on the surface of LECs.
As important inflammatory cells in the tumor microenvironment, macrophages participate in the entire process of tumor development and evolution and regulate the generation of tumor lymphatic vessels through a series of complicated mechanisms. Macrophages can produce multiple cytokines to promote the proliferation, migration and ring formation of peri-tumor LECs. On the other hand, hyperplastic lymphatic vessels may stimulate the aggregation of macrophages into tumor tissues [16]. Barbera-Guillem et al. found that LYVE-1+ macrophages were adjacent to LECs in metastatic melanoma, and VEGF-C expression was identified in macrophages, suggesting that TAMs expressing VEGF-C can promote the generation of tumor lymphatic vessels, phagocytose cell fragments and form tumor-associated immune complexes [17]. In a study of cervical cancer, Schoppmann S. F. et al. found that VEGF-C produced by TAMs had major promotional effects on the generation of lymphatic vessels [18]. In the present study, M2 macrophages were also able to produce VEGF-C and act on VEGFR-3, a surface receptor of LECs, thus promoting the generation of lymphatic vessels. These results suggest that tumor cells and macrophages have similar mechanisms in regulating lymphatic cell production via the VEGF-C/VEGFR-3 signaling pathway but whether other regulating mechanisms exist in these two type of cells that may be present in the complicated microenvironment in vivo remains to be investigated.
It has been shown that cytokines produced by tumor cells and macrophages can induce the generation of tumor-associated lymphatic vessels, while hyperplastic LECs may promote the propagation of tumor cells to lymphatic vessels and the aggregation of macrophages [19]. The current clinical therapy for tumors is the excision of the malignancy supplemented with targeted radiotherapy or chemotherapy. However, these adjuvant therapies are accompanied with serious side effects and may affect normal physiological functions of the body. Regional lymph node metastasis through the lymphatic system may occur in a substantial number of tumors after excision, unfavorably affecting patient prognosis. It has been reported that blocking the VEGF-C/VEGFR-3 signal transduction pathway may inhibit the generation of tumor-associated lymphatic vessels, thus preventing lymph node metastasis of the tumor [20,21]. For therapeutic interventions of tumor-bearing animal models with multiple malignancies, Blance et al. found that by using SAR131675, a new type of VEGFR-3 inhibitor, regional lymph node invasion and metastasis of the malignant tumor could be effectively inhibited without severe side effects, especially in the 4T1 tumor-bearing mouse model of breast cancer, and the inhibitory efficacy on lymph node metastasis reached 50% [22,23]. In the present study, simultaneous inhibition of VEGF-C secretion and expression in tumor cells and macrophages synergistically inhibited the generation of lymphatic vessels, providing a new target for tumor therapies against lymph node metastasis. Of course, this therapeutic idea is not limited to VEGF-C and lymphatic metastasis; similar effects may also exist in blood vessel hyperplasia and metastasis. Meanwhile, the operative excision of tumor tissues with adjuvant radiochemotherapy and synergistic inhibition of lymphatic vessel growth factor production in tumor cells and macrophages may effectively impede the generation of tumor lymphatic vessels in ESCC and other malignancies; this method may provide experimental evidence and theoretical support for gene therapy targeting the inhibition of lymphatic vessel generation and may be an important tool for inhibiting post-operation metastasis of tumors (especially ESCC) and improving patient survival rates.
Acknowledgements
This work was supported by the Foundation and research in cutting-edge technologies in the project of Henan province (52300410026) and by key technology research project of Henan province (132102310086), National Natural Science Foundation of China (81570199).
Disclosure of conflict of interest
None.
References
- 1.Luo LN, He LJ, Gao XY, Huang XX, Shan HB, Luo GY, Li Y, Lin SY, Wang GB, Zhang R, Xu GL, Li JJ. Evaluation of preoperative staging for esophageal squamous cell carcinoma. World J Gastroenterol. 2016;22:6683–6689. doi: 10.3748/wjg.v22.i29.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wroblewski LE, Peek RM Jr, Coburn LA. The Role of the Microbiome in Gastrointestinal Cancer. Gastroenterol Clin North Am. 2016;45:543–556. doi: 10.1016/j.gtc.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Ji X, Cai J, Chen Y, Chen LQ. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J Gastrointest Surg. 2016;8:90–94. doi: 10.4240/wjgs.v8.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Chen Y, Zhang L, Xing L, Xu H, Wang Y, Shi Q, Liang Q. Total saponins of panaxnotoginseng promotes lymphangiogenesis by activation VEGF-C expression of lymphatic endothelial cells. J Ethnopharmacol. 2016;193:293–302. doi: 10.1016/j.jep.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morfoisse F, Renaud E, Hantelys F, Prats AC, Garmy-Susini B. Role of hypoxia and vascular endothelial growth factors in lymphangiogenesis. Mol Cell Oncol. 2015;27:e1024821. doi: 10.1080/23723556.2015.1024821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Caballero M, Blacher S, Paupert J, Quesada AR, Medina MA, Noël A. Novel application assigned to toluquinol: inhibition of lymphangiogenesis by interfering with VEGF-C/VEGFR-3 signalling pathway. Br J Pharmacol. 2016;173:1966–1987. doi: 10.1111/bph.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura Y, Sumiyoshi M, Baba K. Antitumor and antimetastatic activity of synthetic hydroxystilbenes through inhibition of lymphangiogenesis and M2 macrophage differentiation of tumor-associated macrophages. Anticancer Res. 2016;36:137–148. [PubMed] [Google Scholar]
- 9.Ou JJ, Wei X, Peng Y, Zha L, Zhou RB, Shi H, Zhou Q, Liang HJ. Neuropilin-2 mediates lymphangiogenesis of colorectal carcinoma via a VEGFC/VEGFR3 independent signaling. Cancer Lett. 2015;358:200–209. doi: 10.1016/j.canlet.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Kim C, Kim MJ, Schwendener RA, Alitalo K, Heston W, Kim I, Kim WJ, Koh GY. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer. 2011;10:36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 12.Witte D, Thomas A, Ali N, Carlson N, Younes M. Expression of the vascular endothelial growth factor receptor-3 (VEGFR-3) and its ligand VEGF-C in human colorectal adenocarcinoma. Anticancer Res. 2002;22:1463–1466. [PubMed] [Google Scholar]
- 13.Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97:457–464. doi: 10.1002/cncr.11073. [DOI] [PubMed] [Google Scholar]
- 14.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 15.Bry M, Kivelä R, Holopainen T, Anisimov A, Tammela T, Soronen J, Silvola J, Saraste A, Jeltsch M, Korpisalo P, Carmeliet P, Lemström KB, Shibuya M, Ylä-Herttuala S, Alhonen L, Mervaala E, Andersson LC, Knuuti J, Alitalo K. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation. 2010;122:1725–1733. doi: 10.1161/CIRCULATIONAHA.110.957332. [DOI] [PubMed] [Google Scholar]
- 16.Ji RC. Macrophages are important mediators of either tumor- or inflammation- induced lymphangiogenesis. Cell Mol Life Sci. 2012;69:897–914. doi: 10.1007/s00018-011-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002;62:7042–7049. [PubMed] [Google Scholar]
- 18.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohhashi T, Kawai Y. Proposed new lymphology combined with lymphatic physiology, innate immunology, and oncology. J Physiol Sci. 2015;65:51–66. doi: 10.1007/s12576-014-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villaume K, Blanc M, Gouysse G, Walter T, Couderc C, Nejjari M, Vercherat C, Cordier-Bussat M, Roche C, Scoazec JY. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology. 2010;91:268–728. doi: 10.1159/000289569. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y, Hu J, Ma J, Feng K, Zhang X, Yang S, Wang W, Zhang J, Zhang Y. RNAi-mediated silencing of VEGF-C inhibits non-small cell lung cancer progression by simultaneously downregulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent axes-induced ERK, p38 and AKT signalling pathways. Eur J Cancer. 2011;47:2353–2363. doi: 10.1016/j.ejca.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Alam A, Blanc I, Gueguen-Dorbes G, Duclos O, Bonnin J, Barron P, Laplace MC, Morin G, Gaujarengues F, Dol F, Hérault JP, Schaeffer P, Savi P, Bono F. SAR131675, a potent and selective VEGFR-3-TK inhibitor with antilymphangiogenic, antitumoral, and antimetastatic activities. Mol Cancer Ther. 2012;11:1637–1649. doi: 10.1158/1535-7163.MCT-11-0866-T. [DOI] [PubMed] [Google Scholar]
- 23.Espagnolle N, Barron P, Mandron M, Blanc I, Bonnin J, Agnel M, Kerbelec E, Herault JP, Savi P, Bono F, Alam A. Specific inhibition of the VEGFR-3 tyrosine kinase by SAR131675 reduces peripheral and tumor associated immunosuppressive myeloid cells. Cancers (Basel) 2014;6:472–490. doi: 10.3390/cancers6010472. [DOI] [PMC free article] [PubMed] [Google Scholar]