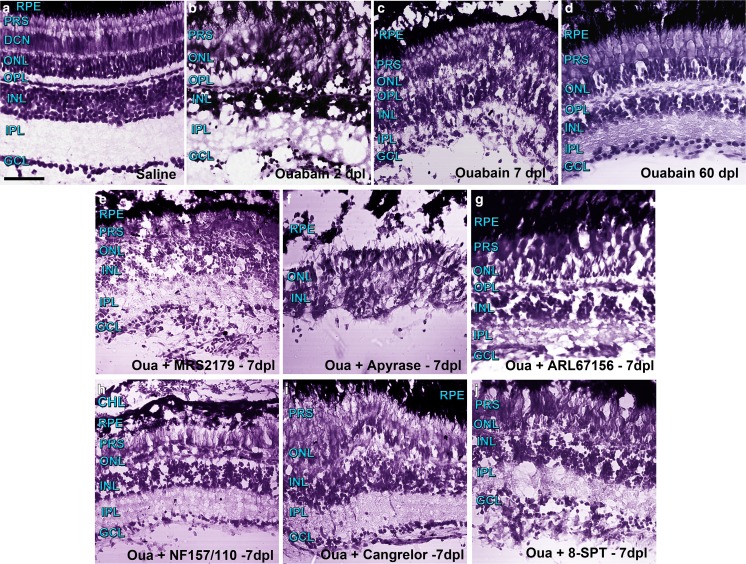
Fig. 1.
Representative photomicrographs of retina sections from zebrafish labelled with Nissl staining were captured with a confocal microscope using differential interference contrast (DIC) filters and the transmitted irradiance detector channel. Groups of five to six zebrafish underwent injections within the vitreous chamber with saline solution (a) or a solution containing 6 μM ouabain on day 0 and were kept alive for 2 (b), 7 (c), and 60 (d) days after lesion (dpl). Other groups of zebrafish were injured in the same manner and received intravitreal treatments with different compounds daily for 6 days starting on day 1 after injury. Control groups of zebrafish (from which images are not shown) underwent intravitreal injections with saline solution, other vehicles such as dimethyl sulfoxide, or inactivated apyrase also for 6 days. Zebrafish were euthanized 7 days after injury to obtain retina sections treated with the following: e 1 μM MRS2179 (antagonist of ADP-activated P2RY1), f 6 U/ml apyrase (ecto-nucleotidase), g 60 μM ARL67156 (ecto-ATPase inhibitor), h 5 μM NF157 plus 5 μM NF110 (antagonists of ATP-activated P2RX3, P2RX2, P2RX1, and P2RY11), i 5 μM cangrelor (antagonist of ADP-activated P2RY12 and P2RY13), or j 11 μM 8-SPT (antagonist of adenosine-activated P1R). Scale bar 40 μm. RPE retinal pigmented epithelium, CH choroid layer, PRS photoreceptor segments, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer