Abstract
Kimura’s disease is a rare disorder of uncertain aetiology seen in Asian population, and presents as painless lymphadenopathy or subcutaneous masses in head-neck region. Peripheral eosinophilia, elevated levels of serum IgE, lymphoid proliferation and eosinophilic infiltration are its characteristic pathological features. We report a case of 40 year old male patient who presented with swelling behind right ear after successful ear surgery. The diagnosis was confirmed by histopathology and was treated by oral steroids.
Keywords: Kimura disease, Angiolymphoid hyperplasia, Eosinophilia, Lymphadenopathy
Introduction
Kimura’s disease is a rare benign chronic inflammatory disorder of unknown aetiology which commonly presents as painless lymphadenopathy or subcutaneous masses in head and neck region. The first report of Kimura disease was from China in 1937, in which Kimm and Szeto described seven cases of a condition they termed “eosinophilic hyperplastic lymphogranuloma” [1]. The disorder received its current name in 1948, when Kimura et al. noted the vascular component and referred to it as an “unusual granulation combined with hyperplastic changes in lymphoid tissue” [2]. The exact prevalence of Kimura’s disease is not known. Most cases of this rare disease are reported in East and Southeast Asia, with a small number of cases reported in Europe. Male to female ratio ranges from 3.5:1 to 9:1 in most series reported, with some exceptions Kimura’s disease is usually seen in young adults during the third decade of life, with the median age being 28–32 years [3, 4]. There have been case reports from various parts of India, but there is hardly any report from Himalayan region. Here we present a sporadic case from this region, which developed the swelling behind the ear after ear surgery.
Case Report
A 40 year old male patient presented with chief complaints of swelling behind right ear since 6 years. He had undergone modified radical mastoidectomy in right ear for squamous variety of chronic suppurative otitis media 6 years back. Though the symptoms of ear disease were relieved after surgery, the patient noticed a swelling behind the operated ear 6 months later. There was no associated neck swelling, puffiness or swelling around eyes or generalized body edema. On clinical examination a swelling of 3 × 4 cm size was found behind the right ear (Fig. 1). It was firm in consistency, fixed to the skin which showed black discoloration, was non-tender, there was no local rise of temperature, and a post aural incision scar was present. The otoscopic examination showed a well epithelialized post-operative cavity in the right ear. There was no other significant finding on systemic or local examination.
Fig. 1.
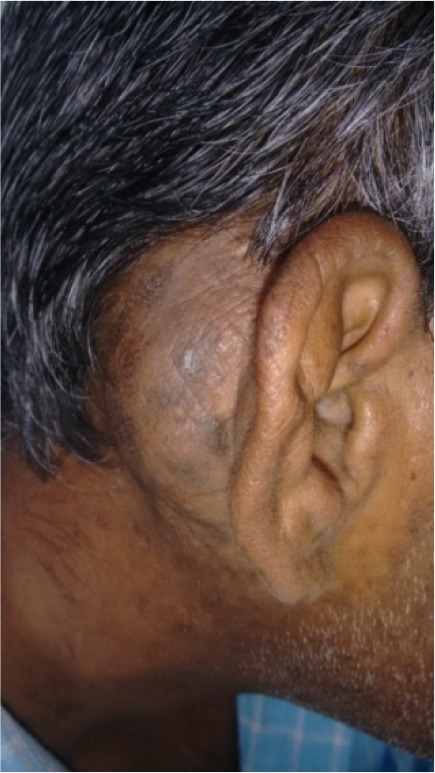
The clinical picture showing swelling behind the ear
Haemogram revealed mild leucocytosis with 11.7% eosinophils and an absolute eosinophil count of 1462 cells/mm3. The erythrocyte sedimentation rate was 25 mm 1st hour. Biochemical parameters including plasma glucose, serum urea, creatinine, bilirubin and liver function tests were found to be within normal range. Urine routine and microscopic examination showed no abnormality. Chest radiography findings were normal. Fine needle aspiration cytology (FNAC) from the post auricular swelling showed a polymorphous population of lymphoid cells comprised of mature lymphocytes, centrocytes, centroblasts, immunoblasts and plasma cells. Numerous eosinophils were seen admixed with the reactive lymphoid cells (Fig. 2). Few histiocytic aggregates and large multinucleated giant cells were also seen. The differential diagnoses of Kimura disease and Angiolymphoid hyperplasia with eosinophilia were suggested on cytology. Incisional biopsy from swelling revealed a lesion composed of lymphoid aggregates with dense eosinophilic infiltrates, in the lower dermis and subcutaneous tissue. Few germinal centres and occasional eosinophilic abscesses were also seen (Fig. 3). The interfollicular areas showed high vascularity and with perivascular infiltrate rich in lymphocytes and eosinophils (Fig. 4). The overlying dermis showed mild acanthosis and subcutaneous tissue showed fibrosis. A diagnosis of Kimura disease was rendered on histopathology. The patient was started on oral prednisone, 60 mg once daily for 2 weeks and then the dose was tapered for 6 weeks. The swelling was significantly reduced after 8 weeks of treatment.
Fig. 2.
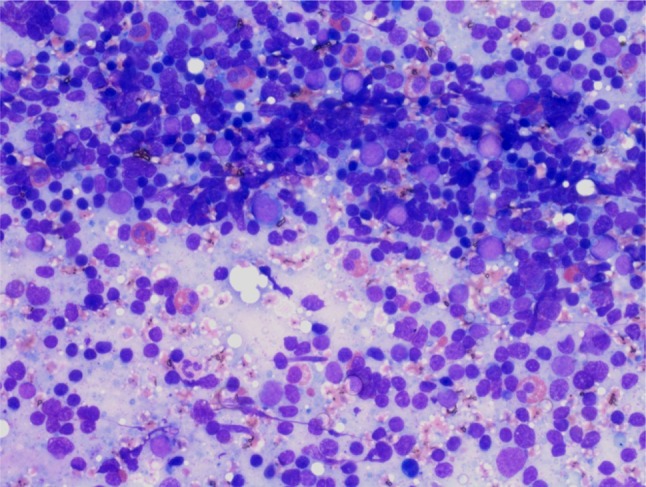
FNAC from the swelling showed a polymorphous population of reactive lymphoid cells admixed with numerous eosinophils. (Giemsa stain, ×400)
Fig. 3.
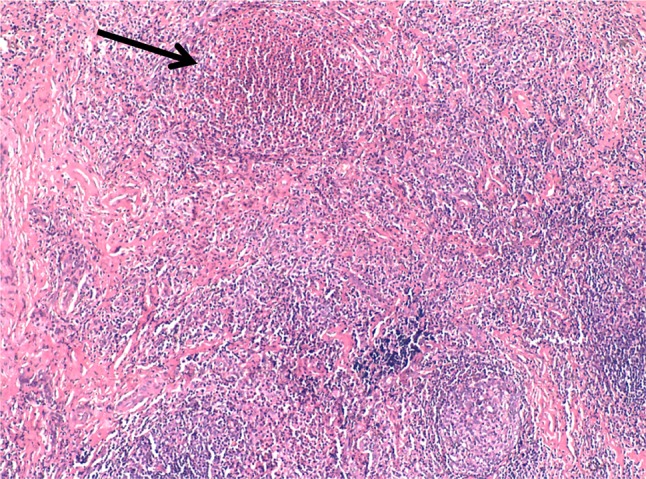
Section shows multiple lymphoid follicles with germinal centres, with prominent vascularization. An eosinophilic abscess is also seen (arrow). (H&E stain, ×100)
Fig. 4.
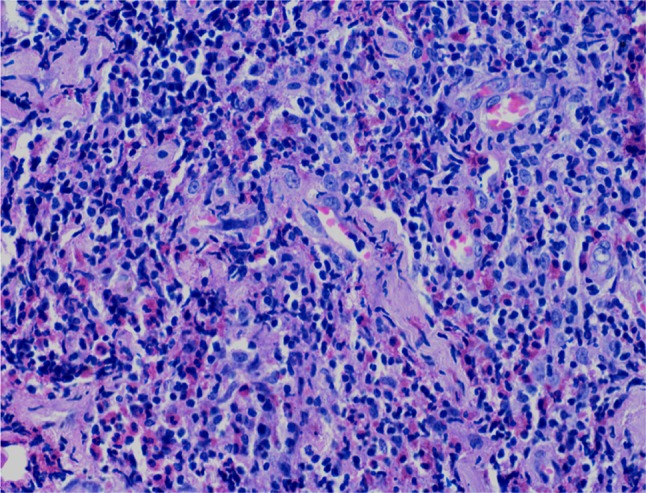
Interfollicular areas showing high vascularity with high endothelial venules and focal hyalinization. The inflammatory infiltrate is comprised predominantly of lymphocytes and many admixed eosinophils. (H&E stain, ×400)
Discussion
The Kimura’s disease is considered as a chronic nonspecific lymphadenitis with presence of numerous eosinophilic infiltrate also called as “eosinophilic lymphadenitis” [5]. The disorder received its current name in 1948, when Kimura’s et al. noted the vascular component and referred to it as an “unusual granulation combined with hyperplastic changes in lymphoid tissue” [2]. The aetiology of Kimura’s disease remains unknown. It has been hypothesized that an infection or toxin may trigger an autoimmune phenomenon or lead to a type I [Immunoglobulin E (IgE)–mediated] hypersensitivity reaction [6].
Kimura’s disease typically presents as a painless mass or masses or subcutaneous nodules in the head and neck region, with occasional pruritus of the overlying skin. These lesions are often associated with lymphadenopathy. Renal disease, nephrotic syndrome in particular, is present in up to 20% of patients with Kimura’s disease [7]. Less commonly, several reports in the literature have linked Kimura’s disease with a hypercoagulable state without associated nephrotic syndrome [8]. Less frequently, the orbit including the eyelids, conjunctiva, and lacrimal glands, paranasal sinuses, epiglottis, tympanic membrane, parotid gland, and parapharyngeal space may be involved [9–11]. Although Kimura’s disease mainly affects the head and neck, involvement of the extremities and inguinal lymph nodes has been reported [12]. In addition, a presentation of Kimura’s disease as a pulmonary hilar mass has recently been described [13].
Nearly all patients with Kimura’s disease demonstrate peripheral blood eosinophilia and elevated levels of serum IgE. Blood urea nitrogen, creatinine, and urinary protein levels should be obtained to exclude concomitant renal dysfunction. Serum eosinophil cationic protein levels parallel the course of the disease [14]. Incisional biopsy is recommended to obtain the diagnosis. Histopathological examination reveals characteristic lymphoid aggregate with prominent germinal center, and marked eosinophilic infiltrate and occasional eosinophilic abscesses. Hyalinized vessels and post capillary vessels are also seen [5]. The lesion can involve the sub cutaneous and deep soft tissue. The closest differential diagnosis includes Angio-Lymphoid Hyperplasia with Eosinophilia (ALHE). The two entities are distinguished on the basis of chemical and histopathological characteristics. ALHE occurs in the older males, and the vascular proliferation is more prominent, with plump endothelial cells [3].
There is no definitive protocol for treatment of Kimura’s disease. Observation is acceptable if the lesions are neither symptomatic nor disfiguring. Though oral corticosteroids are most commonly used, intra-lesional corticosteroids like triamcinolone may be effective for localized disease. Steroids decrease inflammation by suppressing migration of polymorphonuclear leukocytes and reversing capillary permeability. Cyclosporine, oral pentoxifylline, trans-retinoic acid with prednisone, intravenous immunoglobulins, Imatinib, photodynamic therapy have been reported to induce remission. The disease however, frequently recurs after cessation of therapies. Radiotherapy has also occasionally been used to treat recurrent or persistent lesions. However, caution is required in using radiation for cases other than that of recurrent disease and disfiguring lesions. Conservative surgical excision has been considered the treatment of choice for Kimura’s disease in prominent places, though recurrence after surgery is also frequently observed [15].
Conclusion
This case reported here is exceptional as; there are hardly any case reports of Kimura’s disease from Himalayan region; second, this case presented after ear surgery with a post aural incision scar which could have been confused for a hypertrophic scar and incision could also be a triggering factor for the lesions to appear; third, patients from hilly areas who present with peripheral eosinophilia can be mistaken for tropical eosinophilia syndrome or high altitude related eosinophilia in this region, so they should undergo thorough head and neck checkup to rule out Kimura’s disease. We also emphasize that the prognosis of lesion is good and the patients require a long fallow up to check recurrence.
Compliance with Ethical Standards
Conflict of interest
All the authors declare that there is no conflict of interest.
Human and Animal Rights
This article does not contain any studies which experiments with human participants or animals and all institutional and international ethical standards have been fallowed.
Informed consent
Informed consent was obtained from all individual participants included in the study
Contributor Information
Manu Malhotra, Email: manumalhotrallrm@gmail.com.
Saurabh Varshney, Email: drsaurabh68@gmail.com.
Neha Singh, Email: nehasingh.path@gmail.com.
Sushant Kumar, Email: shushantsky@gmail.com.
References
- 1.Rao K, Kumar S (2014) Kimura’s Disease—A Rare Cause of Head and Neck Swelling. Int J Otolaryngol Head Neck Surg 3:200–204
- 2.Kimura T, Yoshimura S, Ishikawa E. On the unusual granulation combined with hyperplastic changes of lymphatic tissues. Trans Soc Pathol Jpn. 1948;37:179–180. [Google Scholar]
- 3.Ramchandani PL, Sabesan T, Hussein K. Angiolymphoid hyperplasia with eosinophilia masquerading as Kimura disease. Br J Oral Maxillofac Surg Jun. 2005;43(3):249–252. doi: 10.1016/j.bjoms.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Sun QF, Xu DZ, Pan SH, et al. Kimura disease: review of the literature. Intern Med J. 2008;38(8):668–672. doi: 10.1111/j.1445-5994.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 5.Juan R (2011) Lymph nodes. In: Rosai and Ackerman’s surgical pathology, 10th edn. Elsevier, Chapter 21, 1805
- 6.Ohta N, Fukase S, Suzuki Y, Ito T, Yoshitake H, Aoyagi M. Increase of Th2 and Tc1 cells in patients with Kimura’s disease. Auris Nasus Larynx. 2011;38(1):77–82. doi: 10.1016/j.anl.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Rajpoot DK, Pahl M, Clark J. Nephrotic syndrome associated with Kimura disease. Pediatr Nephrol. 2000;14(6):486–488. doi: 10.1007/s004670050799. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Hong Y-S. Kimura disease complicated with bowel infarction and multiple arterial thromboses in the extremities. J Clin Rheumatol. 2014;20:38–41. doi: 10.1097/RHU.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 9.Yoganathan P, Meyer DR, Farber MG. Bilateral lacrimal gland involvement with Kimura disease in an African American male. Arch Ophthalmol. 2004;122(6):917–919. doi: 10.1001/archopht.122.6.917. [DOI] [PubMed] [Google Scholar]
- 10.Park SW, Kim HJ, Sung KJ, Lee JH, Park IS. Kimura disease: CT and MR imaging findings. AJNR Am J Neuroradiol. 2012;33(4):784–788. doi: 10.3174/ajnr.A2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonfils P, Moya-Plana A, Badoual C, Nadéri S, Malinvaud D, Laccourreye O. Intraparotid Kimura disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130(2):87–89. doi: 10.1016/j.anorl.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Yadla M, Sriramnaveen P, Sivakumar V, Sandeep Reddy Y, Sridhar AV, Krishna Kishore C. Epitrochlear mass in a patient on maintenance hemodialysis-Kimura disease. Hemodial Int. 2012;16(4):568–570. doi: 10.1111/j.1542-4758.2011.00660.x. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Li YJ, Zhan FH, Dang CJ. The false-positive finding of left pulmonary Kimura disease on 18F-FDG PET/CT. Clin Nucl Med. 2013;38(7):569–572. doi: 10.1097/RLU.0b013e3182867d70. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto M, Komura A, Nishimura S. Hematoserological analysis of Kimura’s disease for optimal treatment. Otolaryngol Head Neck Surg. 2005;132(1):159–160. doi: 10.1016/j.otohns.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Piette EW, James JA (2014) Kimura disease clinical presentation. Medscape. http://emedicine.medscape.com/article/1098777-clinical#b4. Accessed 26 June 2007


