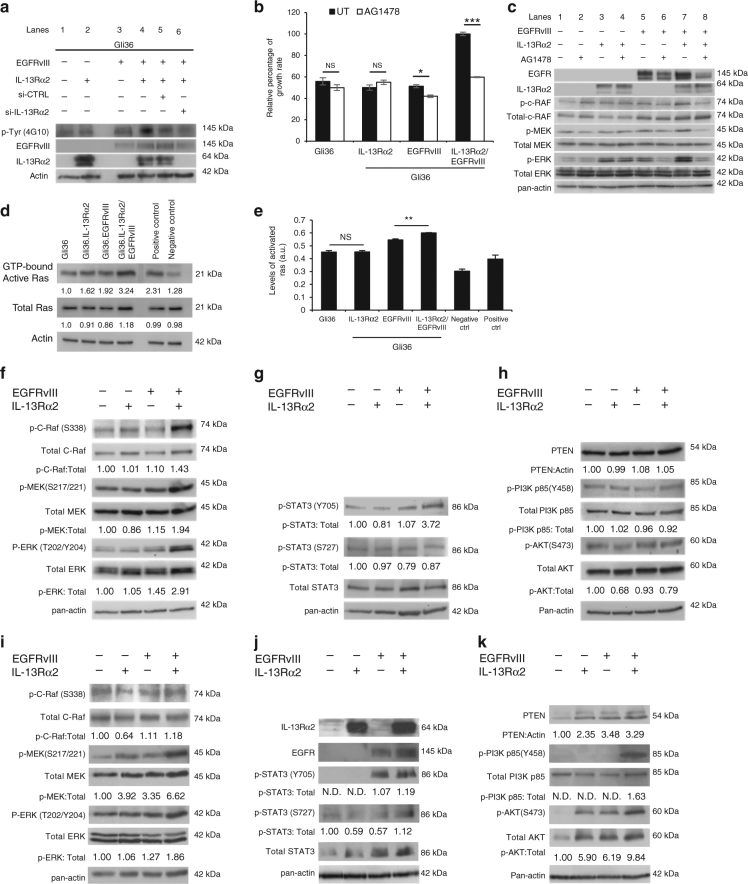
Fig. 4.
Oncogenic signaling of IL-13Rα2 increases tyrosine kinase activities and promote cell proliferation through activation of the RAS/RAF/MEK/ERK signaling cascade. a Cell lysates from Gli36, Gli36.IL-13Rα2, Gli36.EGFRvIII, and Gli36.EGFRvIII/IL-13Rα2 post-knockdown with scrambled or IL-13Rα2 siRNA were examined for the total levels of tyrosine phosphorylation using anti-phosphotyrosine antibodies. b CCK-8 proliferation assay was performed with and without 10 μM AG1478 treatments. All data are represented as mean ± SEM. Unpaired t-test ***p < 0.001 c Endogenous expression levels of the RAS/RAF/MEK/ERK signaling were examined in the indicated cells with and without AG1478 treatment. RAS activation was determined by either d Raf-1 RBD agarose beads pull-down assay or e ELISA assay. Endogenous protein expression levels of f Total and p-C-RAF, Total and p-MEK/p-ERK g Total and p-STAT3 h PTEN, total and p-PI3K p85α and total and p-AKT were examined in the indicated cells. Normal human astrocytes transfected with EGFRvIII, IL-13Rα2 or co-expressing both receptors were examined for endogenous expression levels of i Total and p-C-RAF, Total and p-MEK/p-ERK, j Total and p-STAT3, k PTEN, total and p-PI3K p85α and total and p-AKT. For all immunoblots, pan-actin served as internal loading controls, and band densitometry quantifications for the proteins were performed using ImageJ (NIH). The value derived from densitometry quantification is obtained from normalizing each of the signaling proteins against actin in a single experiment, and presented as a ratio of phosphorylate form over total protein. Each of these was performed at least two independent times