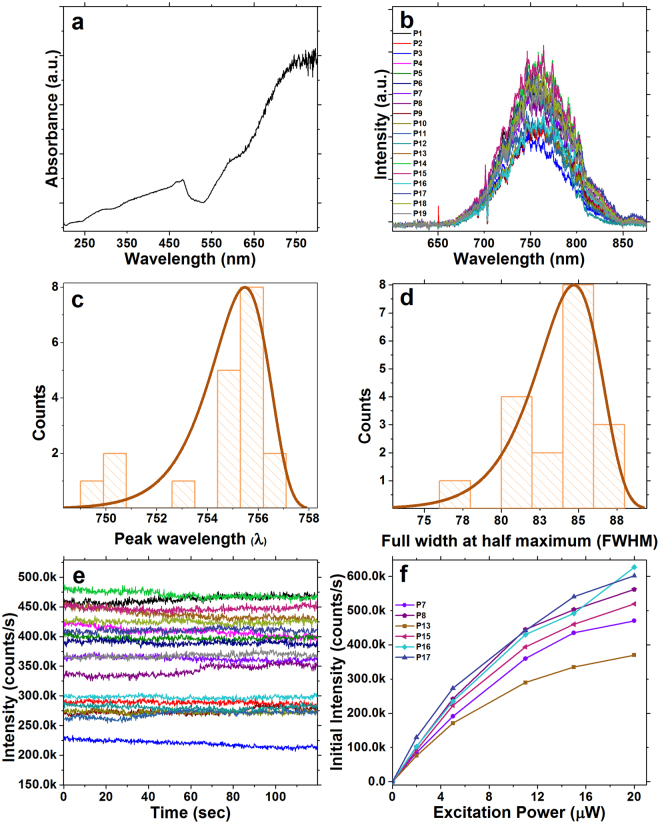
Figure 3.
(a) UV-visible absorbance spectrum of Cu2O nanocubes in water. (b) Fluorescence emission spectra of 19 individual Cu2O nanocubes excited at 520 nm with a supercontinuum picosecond pulsed laser. (c) Emission peak wavelength distribution. The emission peak centered at 754.6 ± 2 nm which can be correlated to oxygen vacancy (Vo). This wavelength is well suited for biological imaging applications. (d) The peak in the distribution of full width at half-maximum (FWHM) emission is around 85 nm. (e) Fluorescence intensity of the same nanocubes as in (a) over a 120 second time period of continuous excitation with 11 μW time-averaged power at the sample from the supercontinuum pulsed laser. Emission intensities of these individual particles ranged between 226 k to 780 k counts/s. (f) Fluorescence emission intensity of 6 selected individual Cu2O nanocubes under 2 μW, 5 μW, 11 μW, 15 μW and 20 μW excitation power. The selected nanocubes in (f) are a subset, chosen for no particular reason, of those studied in (b) and (e). This result indicates that individual Cu2O nanocubes have the ability to produce considerably bright emission while using low excitation powers.