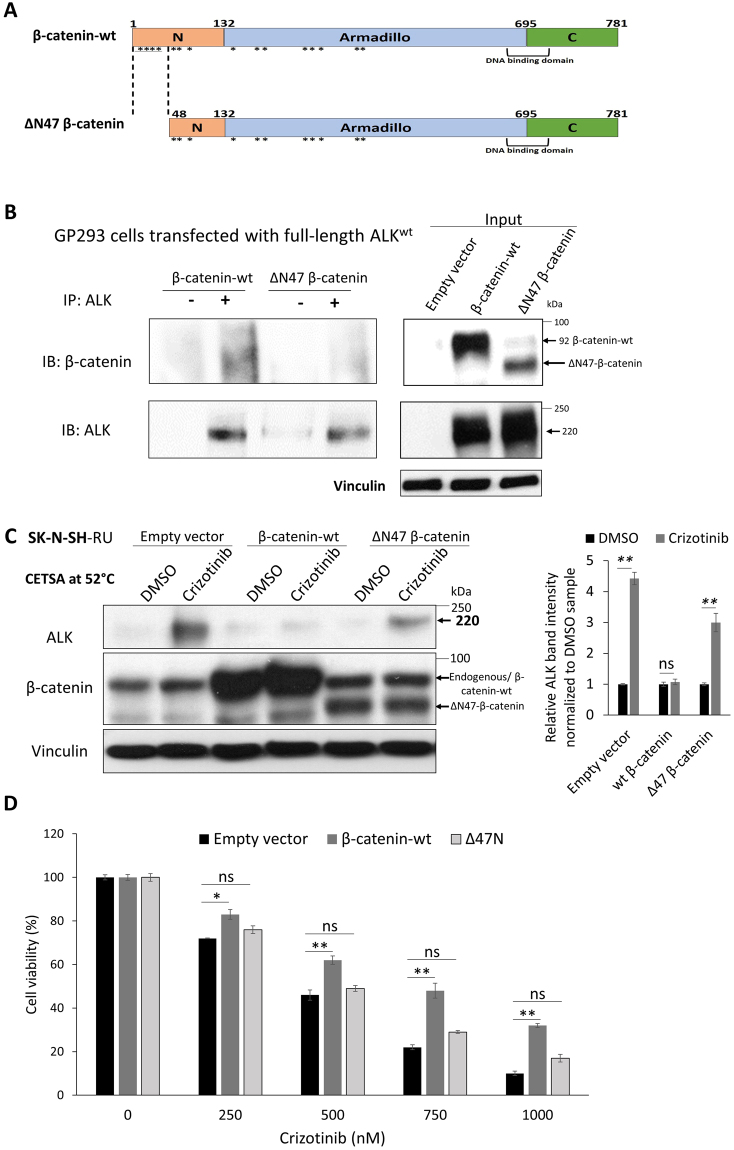
Figure 5.
Enforced β-catenin-wt interacts with ALK and abrogates crizotinib—ALK binding in SK-N-SH-RU cells. (A) Diagram of the two β-catenin plasmids used in this study. Locations of computationally predicted β-catenin residues interacting with ALK (16 residues, *). (B) ALK pull-down was performed using co-immunoprecipitation assay in GP293 cells that were transfected with full-length ALK and with either β-catenin-wt or ΔN47-β-catenin. Full-length ALK co-immunoprecipitated with wild-type β-catenin but not ΔN47 β-catenin. Another exposure for the co-immunoprecipitation blots are presented in Supplementary Figure 5. (C) CETSA was performed to compare crizotinib—ALK binding ability in RU that were transfected with either empty vector, β-catenin-wt or ΔN47-β-catenin. Cells were treated with DMSO or 500 nM crizotinib for 6 hours. Representative ALK western blots are shown on the left panel. CETSA assay was performed at 52 °C. Vinculin level was blotted as a loading control. (D) Enforced expression of β-catenin-wt but not ΔN47 significantly affect crizotinib sensitivity in RU cells in comparison to empty vector transfected cells. All data are presented as mean ± SD, *P < 0.05, **P < 0.01, Student’s t test.