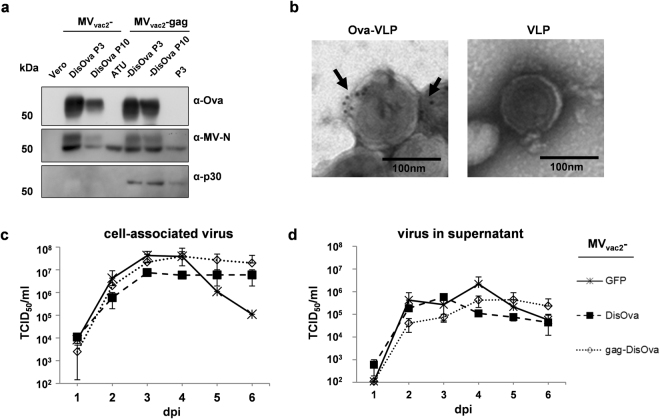
Figure 2.
Characterization of Ovalbumin-presenting recombinant MVvac2. (a) Immunoblot analysis of Vero cells infected at an MOI of 0.03 with MVvac2 or MVvac2-gag expressing membrane-bound extracellular version of Ovalbumin (DisOva) compared to vaccine expressing no antigen (MVvac2-ATU) in virus passages 3 (P3) or 10 (P10). Uninfected Vero cells served as control. Blots were probed as indicated. (b) Immunoelectron microscopic analysis of MLV-derived VLPs purified from supernatants of virus-infected Vero cells. VLPs displaying Ovalbumin or pure VLPs, as depicted above images, analyzed after fixation and probing for Ova. Arrows depict Ova-specific labelling. Scale bar: 100 nm. (c,d) Growth kinetics of recombinant MV (P3) on Vero cells infected at an MOI of 0.03 with indicated viruses. Cell-associated virus titers (c) as well as virus titers in the supernatant (d) of samples prepared at indicated time points post infection were titrated on Vero cells. Means and standard deviations of three independent experiments. dpi, days post infection; TCID50, tissue culture infectious dose 50.