Abstract
While there is considerable interest in, and good evidence for, the role that parasites play in biological invasions, the potential parallel effects of species introduction on parasite dynamics have clearly received less attention. Indeed, much effort has been focused on how parasites can facilitate or limit invasions, and positively or negatively impact native host species and recipient communities. Contrastingly, the potential consequences of biological invasions for the diversity and dynamics of both native and introduced parasites have been and are still mainly overlooked, although successful invasion by non-native host species may have large, contrasting and unpredictable effects on parasites. This review looks at the links between biological invasions and pathogens, and particularly at crustacean invasions in aquatic ecosystems and their potential effects on native and invasive parasites, and discusses what often remains unknown even from well-documented systems. Aquatic crustaceans are hosts to many parasites and are often invasive. Published studies show that crustacean invasion can have highly contrasting effects on parasite dynamics, even when invasive host and parasite species are phylogenetically close to their native counterparts. These effects seem to be dependent on multiple factors such as host suitability, parasite life-cycle or host-specific resistance to parasitic manipulation. Furthermore, introduced hosts can have drastically contrasting effects on parasite standing crop and transmission, two parameters that should be independently assessed before drawing any conclusion on the potential effects of novel hosts on parasites and the key processes influencing disease dynamics following biological invasions. I conclude by calling for greater recognition of biological invasions’ effects on parasite dynamics, more parasite-focused studies and suggest some potential ways to assess these effects.
Keywords: Invasive species, Spillover, Spillback, Crustacean, Amphipod, Complex life cycle, Parasite transmission
Graphical abstract
Highlights
-
•
A review of the literature on crustacean invasions and parasites was conducted.
-
•
Potential effects of crustacean invasions on parasite dynamics are discussed.
-
•
These effects are dependent on a complex combination of multiple factors.
-
•
More parasite-focus studies should be conducted to clearly assess these effects.
1. Introduction
Biological invasions are a global phenomenon resulting in profound changes in native communities (Simon and Townsend, 2003, White et al., 2006, Nalepa et al., 2009). Invasive species are among the main drivers of biodiversity decline, threatening both the environment and economy (Simberloff et al., 2013). Invasion rate and occurrence are constantly increasing due largely to global trade and travel (Dick and Platvoet, 2000, Keller et al., 2011). Invasive species may affect native species through direct competitive interactions or predation and indirectly through resource exploitation. The impact of invasive species can also extend beyond obvious effects of competition and predation on native species (Snyder and Evans, 2006). For example, pathogens can both influence and be affected by biological invasions. Native parasite communities and dynamics can be impacted by both introduced host and co-introduced parasite species (Telfer and Bown, 2012). However, our understanding of the effects of biological invasions and of the factors influencing invasive species impacts on parasites dynamics is still limited.
Interestingly, the role of pathogens in invasion success and impacts is widely recognized even though the number of studies focusing on pathogens compared to free-living organisms is proportionally small (Tompkins et al., 2011, Lowry et al., 2013). Parasites can play a key role in biological invasions, affecting species interactions and co-existence, and thus influencing the success of invasive species (Drake, 2003, Prenter et al., 2004, Hatcher et al., 2006, Dunn, 2009). Indeed, much focus has been put on how parasites may affect the outcome or impact of invasions through their potential effects on interactions among invasive and native host species (Dunn and Perkins, 2012, Dunn et al., 2012, Dunn and Hatcher, 2015). To put it simply, the effects of parasites in biological invasions have been extensively considered and studied while the consequences of biological invasions on parasite diversity and population dynamics have been and are still mainly ignored. It is widely accepted that parasites mediate invasions, but so too can biological invasions impact parasite communities, populations and dynamics (Telfer and Bown, 2012). For example, depending on the ability of a local, native parasite to survive and undergo transmission in a novel, invasive host species, this host can act as a sink or reservoir for native parasites, thus either decreasing or increasing their overall abundance (Gendron and Marcogliese, 2016). Successful invasion of an ecosystem by non-native host species can thus have contrasting, unpredictable effects on native parasites.
Invasive species are generally assumed to suffer less from infection by local pathogens than native hosts (Dunn and Dick, 1998, Torchin et al., 2002, Genner et al., 2008, Redón et al., 2015). Local parasites are not adapted to the new host immune system thus providing a competitive advantage to the invader over native hosts (Emblidge Fromme and Dybdahl, 2006, Genner et al., 2008). For example, the fish acanthocephalan Pomphorhynchus laevis induces lower immuno-competence and imposes energetic costs only in its native amphipod host; invasive hosts become infected but are not affected like native hosts, thus supporting the idea of a maladaptation of native parasites to invasive hosts (Rigaud and Moret, 2003, Cornet et al., 2010). While there is now clear evidence for invading hosts often evading or being less affected by native parasites, studies focusing on the parasites themselves, whether native or introduced, are rare. Invasive host species can affect native host-parasite dynamics in two opposing ways. If an introduced species becomes a competent host to a native parasite, allowing survival and transmission, it may lead to an increase in parasite abundance and standing crop. If a native parasite is acquired by an invasive host that is unsuitable, preventing parasite survival or transmission, a decrease in parasite population may ensue (Telfer et al., 2005, Kopp and Jokela, 2007, Paterson et al., 2011, Lettoof et al., 2013). Interestingly, recent evidence also shows that pathogen dynamics in invasive host species may evolve over time as parasites adapt to the novel hosts, a parameter that has long been documented and accepted with respect to invasion by free-living species (Van Riel et al., 2003, Krakau et al., 2006, Gendron et al., 2012). The European green crab Carcinus maenas went from harbouring significantly fewer, immature native parasites compared to the native shore crab host (Hemigrapsus oregonensis) to being equally infected and affected as the native host in a matter of months (Torchin et al., 1996). Parasite dynamics following invasion by novel hosts may thus depend on time since invasion, and evolve over time; indeed, introduced species may accumulate native parasites (Guégan and Kennedy, 1993, Torchin and Lafferty, 2009). In this review I will first discuss the importance of and relation between biological invasions and pathogens in aquatic ecosystems. Secondly I will explore how parasites may affect biological invasion processes and vice-versa. Finally, I will present specifically the links between invasive crustaceans and parasite dynamics in aquatic ecosystems. I conclude by suggesting some ways to further our knowledge on this matter.
2. Biological invasions and parasites in aquatic ecosystems
Aquatic ecosystems are threatened by many factors such as urbanization, pollution, climate change and habitat alteration, but also largely by the introduction of non-native species (Gherardi, 2007). Fresh waters, in particular, are under constant threat from alien invasive species around the world (Gherardi et al., 2009, Strayer, 2010). Aquatic ecosystems have been rendered more susceptible to invasion by human activities such as the pet trade, live bait transport, shipping and ballast waters or construction of canals connecting once-isolated river basins (Arundell et al., 2015, Laverty et al., 2015). As a result, alien invasive species are considered to be the third most important threat to aquatic ecosystems (Sala et al., 2000). Macroinvertebrates in general and crustaceans in particular are highly successful aquatic invaders and often have substantial impacts on recipient ecosystems (Dumbauld et al., 2011, Hänfling et al., 2011, Blakeslee et al., 2015, Laverty et al., 2015, Ricciardi, 2015). Aquatic macroinvertebrates are important elements of freshwater ecosystems and also hosts to many parasites (Grabner, 2017). In Europe, they represent around half of invasive freshwater species (Devin et al., 2005, Karatayev et al., 2009, Hänfling et al., 2011). Ponto-Caspian crustaceans, for example, have largely colonized freshwater and brackish ecosystems in Europe and North America (Van den Brink et al., 1991, Ricciardi and MacIsaac, 2000). For amphipods alone, ten new species are now established in central and western Europe (Dick and Platvoet, 2000, Bij de Vaate et al., 2002). All of these are vehicles for a multitude of co-introduced parasites or potential novel hosts to local, native parasites in colonized ecosystems (Hatcher et al., 2015). Non-native free-living species may also alter native parasite dynamics and facilitate the establishment of independently arriving parasite species (Knudsen, 1995, Amundsen et al., 2013). However, changes in native or co-introduced parasite dynamics in areas colonized by novel hosts are mostly unknown and seldom documented unless they negatively affect native and/or invasive host species, thus influencing invasion patterns. Parasites can indeed alter the distribution and abundance of hosts, food-web structure, biomass and ecosystem integrity (Marcogliese and Cone, 1997, Marcogliese, 2005, Lafferty et al., 2006, Lagrue and Poulin, 2016). Changes in parasite dynamics following biological invasions are thus likely to have far-reaching effects on the whole ecosystem and vice-versa (Dumbauld et al., 2011, Amundsen et al., 2013). However, the effects of native parasites on invasive host success have received much more attention than the opposite scenario.
3. Parasitism in aquatic crustaceans
Crustaceans are important components of aquatic ecosystems and hosts to a wide diversity of parasites (Hatcher et al., 2015, Grabner, 2017). Parasites can in turn alter the distribution and abundance of their hosts and significantly influence food-web structure, biomass and ecosystem integrity (Marcogliese and Cone, 1997, Lafferty et al., 2006, Kuris et al., 2008, Lagrue and Poulin, 2016). Parasites often influence the ecological relationships of free-living species and alter species diversity and co-existence (Mouritsen and Poulin, 2002, Hatcher et al., 2006). Potential changes in parasite or host communities through biological invasions may thus have in depth effects on aquatic ecosystem integrity but also parasite dynamics (Mastitsky et al., 2014).
Aquatic crustaceans are hosts to parasites with different life cycles (from single host and direct transmission to complex with trophic transmission) and with highly variable infection levels and pathological effects (Jackson et al., 1997, Wedekind et al., 2000, Stentiford et al., 2013); invasive crustaceans hosting high numbers of potentially harmful parasites (Mastitsky et al., 2014). For example, Gammarus spp. amphipods are hosts to intracellular microsporidians with direct vertical or horizontal transmission and a wide diversity of helminth parasites with complex life cycles such as acanthocephalans, trematodes, cestodes or nematodes (Grabner et al., 2015, Grabner, 2017). Prevalence (i.e. proportion of infected hosts) can vary widely among parasites infecting amphipod crustaceans, from very high (up to 100% in microsporidians) to relatively low for acanthocephalans for example (Kennedy, 2006, Grabner, 2017), and thus strongly influence the likelihood of pathogens being co-introduced with invasive crustacean hosts. Within crustaceans, some taxa are also more likely to be carriers of co-introduced pathogens or recipients of invasive parasites. Planktonic crustaceans in general and copepods in particular for instance are commonly introduced in new areas and hosts to a variety of pathogens including many species of potentially high impacts parasites such as the eel nematode Anguilicolla crassus or Diphyllobothrium spp. cestodes (Milner and Mayer, 1982, Marcogliese, 1995, Mastitsky et al., 2014). Furthermore, some of the most invasive aquatic crustaceans worldwide are hosts to a number of parasites, either co-invading with their original host or encountered in the colonized area. The European green crab, Carcinus maenas, is one of the most common invasive crustaceans worldwide and also one of the most intensively studied. It is host to a number of parasites occurring in varying intensities and prevalences (Torchin et al., 2001, Thieltges et al., 2008). The cirriped Sacculina carcini, which forms conspicuous external brood sacks and castrates the crab host is well known but other parasites, mainly helminths with sea bird final hosts, are also extremely common, both in the native and invasive range of the crab host (Zetlmeisl et al., 2011, Blakeslee et al., 2015). However, native and introduced populations of parasites are hardly ever studied comparatively from an ecological or evolutionary perspective (Taraschewski, 2006).
Parasites can also have severe, but highly variable, pathogenic effects on their crustacean hosts such as increased mortality, castration, phenotypic alterations (Reinhard, 1956, Kuris and Lafferty, 1992, Kennedy, 2006, Lafferty and Kuris, 2009, Gehman and Byers, 2017). Crustacean pathogens can strongly influence host population dynamics, interspecific interactions and community structure (Mastitsky et al., 2014). In the Mediterranean, brine shrimps (Branchiopoda) are intermediate hosts to twelve species of cestode parasites using birds as definitive hosts (Vasileva et al., 2009). Larval cestodes have higher infection levels and pathogenic effects on native brine shrimps than on the invasive species Artemia franciscana (Redón et al., 2015). As a result, avian cestodes are likely to help A. franciscana outcompete native species. At the same time however, the invasive host may impact native parasite dynamics and eventually reduce its own parasite-mediated competitive advantage over native hosts. Overall, changes in parasite diversity in crustacean populations through introduction of new hosts and/or parasites can have large effects on both native and invasive hosts and parasites (Hatcher et al., 2015). Especially if shared parasites differentially infect and/or affect invasive and native hosts (Rode et al., 2013).
With the increasing recognition that parasites play important roles in ecosystem functioning and can influence species coexistence patterns, biological invasions, energy flow and community stability (Hudson et al., 2006, Hatcher et al., 2012a, Hatcher et al., 2012b), the potential effects of biological invasions in parasite dynamics and infection levels in both native and introduced hosts now require further attention and specifically designed studies to assess their full extents. Novel pathogens continue to be co-introduced with invasive hosts and transfer to native species with potentially devastating consequences, yet the population dynamics of this kind of biological invasions remain poorly understood (Woolhouse et al., 2005, Peeler et al., 2011). Below I present the current state of knowledge and highlight areas of missing information about interactions between biological invasions and parasites with a particular focus on aquatic invertebrates and parasite dynamics.
4. Enemy release, dilution effects, spillover and spillback in aquatic invaders
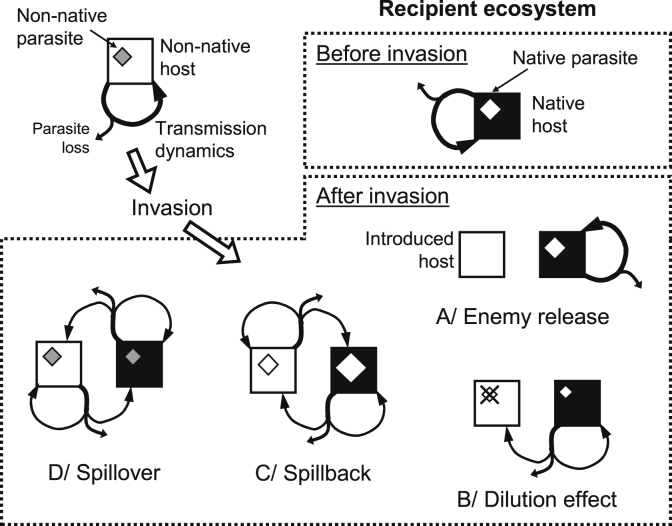
Parasites may affect the establishment and spread of invasive host species in multiple ways. Stochastic effects and the small numbers of hosts present in the early stages of invasion often reduce the diversity of, and sometimes completely eliminate, parasites carried by invasive species (Blakeslee et al., 2012, Firmat et al., 2016). For example, the European green crab (Carcinus maenas), a notorious invader, has escaped more than two-thirds of its native parasite load when colonizing North America and its status as one of the most successful marine invasive species has partly been attributed to the loss of parasites (Zetlmeisl et al., 2011, Blakeslee et al., 2015). Depending on the pathogenic effects of parasites on the host, adverse conditions during invasion may also remove infected hosts, increasing the likelihood of parasite loss. Many other factors such as time since introduction, introduction vector or distance from native range may influence the parasite diversity and prevalence in introduced crustacean hosts (Torchin et al., 1996, Blakeslee et al., 2009). Species introduced via ballast water tend to have the fewest parasites in their introduced ranges. For example, the European green crab, Carcinus maenas, has fewer parasites in regions where it was introduced via ballast water compared to areas colonized through other introduction vectors (Torchin et al., 2001, Torchin et al., 2002). Overall, non-native aquatic species frequently lose parasites upon introduction but in often different ways (Blakeslee et al., 2009). These phenomena can contribute in turn to the success of the invader through enemy release (Keane and Crawley, 2002, Clay, 2003, Torchin et al., 2003, Torchin and Mitchell, 2004, Fig. 1A). However, the probability of parasite loss is highly parasite-specific and depends largely on their mechanism of transmission (Slothouber Galbreath et al., 2004, Hatcher and Dunn, 2011). Furthermore, parasite burdens (richness and loads) and their pathological impacts on invasive hosts compared to their native counterparts can be highly variable, ranging from much lower infection levels to equivalent or higher (Roy et al., 2011, Redón et al., 2015). As a result, parasite dynamics in invasive crustaceans do not always fit the enemy release hypothesis (Roy et al., 2011). For example, invasion of Great Britain by the amphipod Dikerogammarus villosus was accompanied by the loss of its microsporidian parasites despite multiple separate introductions (Arundell et al., 2015), but the invasive populations of the amphipod Crangonyx pseudogracilis show no sign of reduced parasite burden (Slothouber Galbreath et al., 2010).
Fig. 1.
Hypothetical examples of enemy release (A), dilution effect (B), parasite spillback (C) and spillover (D) following introduction of a non-native host in a recipient ecosystem, illustrating the fundamental differences among the different processes. The theoretical recipient ecosystem is here composed of a native host infected by a parasite with a simple life cycle and direct transmission, invaded by a congeneric non-native host infected with a co-introduced parasite with a similar life cycle, to simplify representation. The variable sizes of squares and diamonds represent relative host and parasite abundances, respectively. The thickness of the arrows represents transmission dynamics of the parasite and account for parasite loss during transmission. Enemy release (A) happens when the introduced species benefits from a reduction, or total loss as represented here, in parasitism as a result of invasion. This may in turn have drastic effects on invasion success and both native and invasive host abundances. Dilution effect (B) results from the failure of native parasites to use invasive hosts for successful reproduction and transmission. Native parasites may be unable to infect or be killed (as represented here) by the invasive host. Dilution may in turn decrease parasite transmission among native hosts and negatively affect parasite population dynamics. Parasite spillback (C) happens when invasive hosts acquire a native parasite that is already present in the native host population. Infected invasive hosts can then act as reservoirs of native parasites, potentially increasing infection levels in native hosts as represented here. Increased infection levels in the native host may in turn reduce native host abundance, compared to pre-invasion levels (not represented here). Parasite spillover (D) follows the co-introduction of non-native parasites with their invasive hosts and infection of native hosts by the introduced parasite. Infection of the native host can be maintained by the invasive host, which acts as a reservoir of infection, self-sustained if the parasite can reproduce in its novel host, or both as represented here. Infection of the native host by the introduced parasite can in turn influence host abundances, compared to pre-invasion levels. Note that in scenario D, the native host may or may not possess native parasites.
Native parasites of the local fauna may also be less effective at infecting invading hosts (Ebert, 1994, Kaltz and Shykoff, 1998, Emblidge Fromme and Dybdahl, 2006, Redón et al., 2015). Even if hosts are taxonomically close, parasites are often adapted to their local hosts and may not be able to infect or induce pathogenic effects in the invading host species (Cornet et al., 2010). For example, the invasive American brine shrimp (Artemia franciscana) have consistently lower cestode infection levels than its native counterpart A. salina. Pathogenic effects of infection, especially host castration and manipulation, are much stronger in native than invasive brine shrimps (Redón et al., 2015). The same trend was observed between A. franciscana and another native brine shrimp species (A. parthenogenetica) infected with microsporidian parasites; the invader was four times less likely to be infected compared with native A. parthenogenetica (Rode et al., 2013). Native pathogens are thus often considered to influence competitive interactions between native and invasive hosts, favoring the invader and partly explaining its success (Georgiev et al., 2007, Sánchez et al., 2012). For example, the introduced amphipod Gammarus tigrinus is outcompeted by the native G. d. celticus in direct interactions but infection by native pathogens mitigates direct interactions and allows the smaller and less aggressive invader to coexist with the native species (MacNeil et al., 2003a). Failure of native pathogens to use invasive hosts may also decrease disease transmission among native hosts and negatively affect parasite population dynamics through dilution effects (Krakau et al., 2006, Hall et al., 2009, Kelly et al., 2009b, Ostfeld and Keesing, 2012, Fig. 1B). Invasive hosts effectively become an infection sink for the native parasite (Paterson et al., 2011, Paterson et al., 2013a). Overall, native and invasive hosts dramatically vary in their vulnerability to infection and suitability as transmission vectors and parasites in their host specificity, pathogenic effect, life cycles and transmission strategies (Redón et al., 2015). These combine to make predictions about the potential effects of parasites on novel host invasion success, as well as the effects of novel, invasive hosts on parasite dynamics extremely difficult.
Invasive species that acquire local, native parasites within their extended range may also act as reservoirs for parasite spillback to native hosts (Kelly et al., 2009a, Dunn et al., 2012, Fig. 1C). As shown above, biological invasions often expose parasites to new hosts in which local parasites are less successful (Cornet et al., 2010). However, in some cases, introduced host species are highly competent hosts for local, native parasites (Paterson et al., 2013b). This may have major effects on both the invasion success of these introduced host species and local parasite dynamics. For example, several microsporidian parasites of native amphipods appear capable of infecting D. villosus and spillback of these native parasites from the invasive species may lead to higher infection levels in native host species, thus increasing parasite prevalence and transmission rates (Wattier et al., 2007, Hatcher et al., 2012a). Invasive amphipods are also commonly infected by local acanthocephalan parasites and, although they often suffer lower levels of pathogenic effects and parasite-induced phenotypic changes than the local host species, they may act as a reservoir for these parasites (Lagrue et al., 2007, Tain et al., 2007, Cornet et al., 2010). In rare cases native parasites may even be beneficial to their native hosts by reducing the invader's impacts, mediating interspecific competition and allowing host co-existence (Macneil et al., 2003b, MacNeil and Dick, 2011). However, invasive hosts may also be highly vulnerable to native pathogens and contain a significant proportion of the parasite standing crop but have a very limited influence on parasite population dynamics (Paterson et al., 2013b). The relative densities of native and introduced hosts and their respective suitability for parasites are further key components to consider when evaluating whether invasive host species affect native parasite dynamics.
Finally, parasites carried by invasive species may also spillover and infect native host species (Font and Tate, 1994, Strauss et al., 2012, Ieshko et al., 2013, Fig. 1D). The spread of invasive hosts may create new co-invasive parasites-native host combinations with potentially dramatic effects on native hosts naïve to the new parasites (Ieshko et al., 2013). Invasive crustaceans are important vectors of co-introduced microsporidian parasites, very common intracellular parasites in amphipods (Haine et al., 2007, Wattier et al., 2007, Bacela-Spychalska et al., 2012). For example, Dikerogammarus villosus, one of the most invasive freshwater species worldwide (Bij de Vaate et al., 2002, Bollache et al., 2004, Grabowski et al., 2007, Wattier et al., 2007), is the original host of the microsporidian Cucumispora dikerogammari (Wattier et al., 2007, Ovcharenko et al., 2010). Cucumispora dikerogammari has spread out successfully with its host and has been detected in many invasive D. villosus populations all over Europe (Wattier et al., 2007). As a result, many native amphipod species have acquired the parasite along D. villosus' invasion path (Bacela-Spychalska et al., 2012). However, the parasite's epidemiology in these novel hosts compared to that in the original D. villosus host has never been documented to my knowledge. Generally, if native host species are more vulnerable to the parasite and/or suffer higher pathogenic effects, then parasite dynamics in these novel hosts may be drastically different to that observed in the original host species (Price et al., 1986, Ieshko et al., 2013).
5. Biological invasions, aquatic crustaceans and parasite dynamics
5.1. Introduced host and native parasites
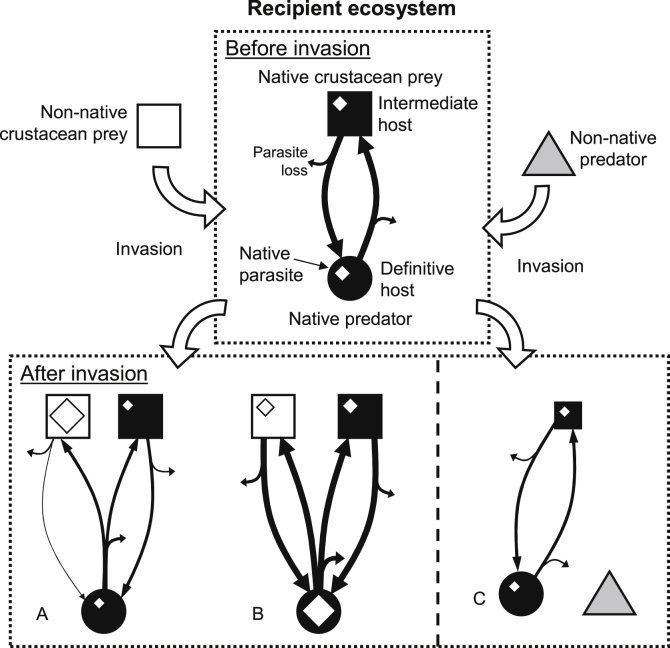
Successful invaders may harbour fewer local parasites than native species in the same community (Roche et al., 2010, Redón et al., 2015). For example, when invasive, the amphipod Gammarus pulex has lower parasite diversity than the native G. duebeni celticus and lower burdens of the two shared parasite species (Dunn, 2009). In extreme cases, when introduced species exclude native host species, parasites specific to these native hosts will be excluded as well (Torchin et al., 2002). Nevertheless, introduced species may acquire native parasites, especially those transmitted through the food chain, and modify native host-parasite dynamics (Paterson et al., 2011, Poulin et al., 2011, Telfer and Bown, 2012, Reisinger and Lodge, 2016, Fig. 2). Invasive hosts acquire about five native parasite species on average, some becoming hosts to more than 16 (Torchin et al., 2003, Kelly et al., 2009b). The majority of invertebrates considered in studies assessing infection status of invasive species acquired some of the local parasites of colonized regions (Mastitsky et al., 2010, Redón et al., 2015). Invasive hosts can subsequently be affected by native parasites in unpredictable ways. For example, trematode parasites (Microphallus spp.) affect invasive crayfish (Orconectes rusticus) growth, behaviour and feeding, altering crayfish impacts on recipient lake communities (Reisinger and Lodge, 2016). However, the potential effects of invasive hosts on native parasite dynamics, transmission probabilities and standing crops are often unknown, most studies being host-focused.
Fig. 2.
Introduced hosts – native parasites: hypothetical examples of the potential effects of invasive crustaceans on native parasites. Note that only a subsample of non-exclusive scenarios from a number of potential outcomes of biological invasion on native parasite dynamics is represented here. The hypothetical native parasite considered here has a two-host life cycle involving a definitive host predator and an intermediate host prey, transmission from the intermediate host to the definitive host requiring consumption of infected intermediate host prey. The variable sizes of squares, circles and diamonds represent relative intermediate and definitive hosts, and parasite abundances, respectively. During transmission, some parasites are unsuccessful and therefore lost from the system (parasite loss); the thickness of the arrows indicates the relative numbers that are either lost or successfully transmitted. The life cycle at the top represents the situation prior to the invasion, providing a benchmark for comparisons. (A) The invader is a suitable alternative intermediate host in which native parasite larvae can survive. However, the introduced host is also a poor transmission vector, due to low predation rate from the definitive host and/or failed host manipulation by the parasite, for example. Introduced hosts are thus more infected than their congeneric, native hosts only because of the accumulation of native parasite larvae that fail to get transmitted to the definitive host. This may in turn negatively affect parasite dynamics in native hosts as shown here. (B) The invader is again a suitable alternative intermediate host but also a good transmission vector to the definitive host, leading to greater infection risk for native definitive hosts. In this case, the invader positively influences parasite dynamics and may increase infection levels in definitive hosts, as shown here. In extreme cases, invasive hosts may be more efficient vectors for the parasite than native hosts and become key hosts. (C) The invader is not a suitable host but directly impacts native intermediate hosts, the transmission vector for the parasite, through predation and thus indirectly reduces native parasite abundance in native definitive hosts.
The acanthocephalan Pomphorhynchus laevis uses a range of amphipod species as intermediate hosts (Kennedy, 2006). In France, many amphipod host communities are made of native Gammarus pulex/Gammarus fossarum and the invasive Gammarus roeseli (Chovet and Lécureuil, 1994, Bauer et al., 2000, Rigaud and Moret, 2003, Lagrue et al., 2007). Pomphorhynchus laevis is able to alter immune defences (Rigaud and Moret, 2003, Cornet et al., 2009) and various behavioural traits (Cézilly et al., 2000, Kaldonski et al., 2007) of the native G. pulex, thus favoring host exploitation and parasite transmission to the definitive fish hosts (Lagrue et al., 2007). Behavioural and physiological alterations of the amphipod host are strongly related to parasite transmission dynamics; both have been shown to be more successful in native hosts than in the invasive G. roeseli (Rigaud and Moret, 2003, Moret et al., 2007, Tain et al., 2007). As a result, although both native and invasive amphipods are used by the parasite, native G. pulex/G. fossarum are more important to the parasite dynamics and can be considered a key host for P. laevis (Bauer and Rigaud, 2015). Key hosts are those contributing significantly more to the completion of the parasite life cycle (Streicker et al., 2013). In the context of biological invasions, whether the native host species consistently remains the key host for its native parasites or congeneric invasive hosts take over this role remains mostly unknown even though this could have deep implications for native parasite dynamics. For example, the amphipod Gammarus pulex is invasive in Ireland where it is often found co-occurring with the native G. duebeni celticus. In mixed-host populations, the fish acanthocephalan parasite Echinorhynchus truttae uses both host species as intermediate host but prevalence is low in the native (0–1%), but high in the invader (up to 70%; MacNeil et al., 2003c). In this case, the invader may have become the key host. However, for parasites with complex life cycles, transmission to the definitive host is a vital component of parasite life history. Intermediate host species that are heavily infected are not necessarily good transmission vectors, and may be more infected than a congeneric host through the accumulation of parasite larvae that fail to get transmitted to their definitive host (Lagrue et al., 2007, Fig. 2A).
Unfortunately, the relative susceptibility of the invasive and native host species to infection is rarely quantified. Yet this information is crucial to evaluate the relative importance of alternative hosts in native parasite dynamics. For example, if native amphipod hosts are more susceptible to infection by local acanthocephalans than the invasive G. roeseli, then both host susceptibility and manipulation maintain native host species as the main route for transmission and parasite dynamics. However, if the invasive G. roeseli proves more susceptible than native amphipod hosts but parasites are not transmitted due to the invader resisting manipulation, then parasite transmission and thus population dynamics should be negatively affected by the invasive host. The acanthocephalan P. laevis is indeed unable to manipulate G. roeseli; this host is thus inefficient at transmitting the parasite (Lagrue et al., 2007). This could in turn impact parasite dynamics while increasing the standing crop of larval parasites in amphipod host populations (Hall et al., 2009, Johnson et al., 2009); the invasive G. roeseli accumulating most of the parasites that are seldom transmitted to definitive hosts (Lagrue et al., 2007, Bauer and Rigaud, 2015, Fig. 2A). Generally, G. roeseli is also less vulnerable to fish predation than native amphipods (Bollache et al., 2006, Kaldonski et al., 2008). Overall, fish parasites using G. roeseli as intermediate hosts have a lower probability of completing their life cycle and, in this case, the invasive host is a dead-end sink negatively affecting parasite dynamics.
However, such patterns are likely parasite-specific. Invasive G. roeseli hosts have been shown to be successfully manipulated by the native bird acanthocephalan, Polymorphus minutus, increasing predation by the appropriate definitive host and increasing transmission (Médoc et al., 2006). In this case, the invader has the potential to become the key host for P. minutus and influence parasite dynamics in a completely contrasting way to the fish acanthocephalan P. laevis (Fig. 2B). Contrastingly, the replacement on the native brine shrimp Artemia salina by a phylogenetically close American species (A. franciscana) may induce the co-extinction of some native bird cestode species using A. salina as intermediate hosts (Redón et al., 2015). Although A. franciscana has acquired a few novel parasites following its introduction, it is possible that some cestodes will not be able to infect this new host at all or at rates sufficient to ensure the survival of a viable population, according to the very low prevalences and the weaker manipulation observed in invasive hosts. However, quantitative estimations of parasite dynamics following native host replacement by the invasive A. franciscana are still required, as is often the case, to assess parasite population viability. Overall, quantitative effects of invasive hosts on native parasite dynamics and population size are difficult to predict and remain undocumented in most cases. Interactions between parasitism and biological invasions are usually considered in cases where invasive species share parasites with their native counterparts in the same community, whether increasing parasite prevalence and abundance or acting as a sink (Prenter et al., 2004, Kopp and Jokela, 2007, Paterson et al., 2011). However, in some cases, invasive host species may replace or create a whole new transmission pathway and may have positive effects on native parasites, in terms of population dynamics or distribution range (Emde et al., 2012, Reisinger and Lodge, 2016).
Finally, invasive species may also indirectly affect native parasites to which they are not actually hosts, by interfering with transmission pathways among hosts (Thieltges et al., 2009, Poulin et al., 2011). By altering the relative abundance of intermediate hosts needed in native parasite life cycles, particularly through predation, invasive species may negatively affect native parasite population dynamics (Poulin et al., 2011, Fig. 2C). For example, the invasive crayfish Pacifastacus leniusculus can decrease native parasite abundance through predation on benthic invertebrates, which act as transmission vectors of many parasites. Native fish hosts from crayfish-infested lakes have significantly fewer parasites transmitted from snails and benthic crustaceans than fish from areas free of P. leniusculus (Fig. 2C). In contrast, parasites transmitted to the same fish hosts from planktonic crustaceans, hosts not preyed upon by the benthic crayfish invader, remain unaffected (Pulkkinen et al., 2013). Indirect effects of invasive species on native parasites are here mediated via effects on native host populations and communities. Overall, effects of invasive host species on native parasites are thus likely to be complex and very rarely accounted for.
5.2. Introduced hosts and introduced parasites
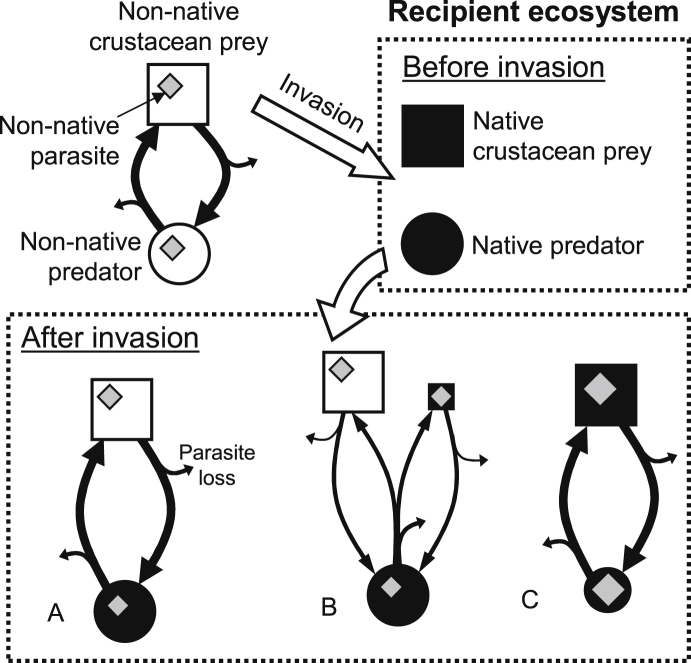
Parasites have frequently been invoked as drivers of invasions, but have received less attention as invasion passengers (Blackburn and Ewen, 2016). Parasites can themselves be introduced and become invasive; most parasites are indeed disseminated by movements of infected hosts (Kennedy, 1993). Almost 80% of parasites known to have been co-introduced with invasive hosts were successful at infecting native hosts, regardless of their life cycle (direct or complex; Font and Tate, 1994, Ieshko et al., 2013, Lymbery et al., 2014, Fig. 3). Evidence also suggests that introduced parasites can alter local, recipient food webs qualitatively and quantitatively through shifts in trophic relationships within the web (Amundsen et al., 2013, Britton, 2013). These changes may in turn influence population dynamics of both introduced and native parasite species (Knudsen, 1995). For example, the microsporidian Cucumispora dikerogammari infecting the invasive amphipod Dikerogammarus villosus has hitchhiked its way into most of Europe with its amphipod host and become a potential emerging disease into invaded areas (Ovcharenko et al., 2010). However, not all parasites are as likely to shift to or be as successful in new hosts (Prenter et al., 2004, Hatcher et al., 2012a). For successful establishment, maintenance and spread, invasive species must be able to survive and reproduce in new ecosystems and host communities (Miller and Ruiz, 2009). Introduced parasites thus need either their original hosts to establish and reproduce or to find alternative suitable hosts. The probability of either or both of these alternatives being met depends on traits relating to parasite life history (Blackburn and Ewen, 2016).
Fig. 3.
Introduced parasites – native/introduced hosts: hypothetical examples of the potential effects of invasive crustaceans on native parasites. Note that only a subsample of non-exclusive scenarios from a number of potential outcomes of non-native parasite introduction is represented here. The hypothetical non-native parasite considered here has a two-host life cycle involving a definitive host predator and an intermediate host prey, transmission from the intermediate host to the definitive host requiring consumption of infected intermediate host prey. The variable sizes of squares, circles and diamonds represent relative intermediate and definitive hosts, and parasite abundances, respectively. During transmission, some parasites are unsuccessful and therefore lost from the system (parasite loss); the thickness of the arrows indicates the relative numbers that are either lost or successfully transmitted. The life cycle at the top left represents the situation in the ecosystem of origin of the parasite, providing a benchmark for comparisons. Prior to the invasion, the hypothetical recipient ecosystem does not contain native parasites for simplification of representation. (A) The parasite is co-introduced with its intermediate host prey. The invasive parasite retains its original, co-introduced hosts and uses native definitive hosts to complete its life cycle. The situation represented here is the simplest one where the native predator exactly replaces the original definitive host of the parasite with no effect on either parasite dynamics or host abundance. However, parasite invasion may in turn negatively affect native predators and change parasite dynamics compared to that observed in the original ecosystem (shown at the top left). (B) The parasite is again co-introduced with its intermediate host prey. The invasive parasite retains its original, co-introduced hosts and uses native definitive hosts to complete its life cycle but also uses the native prey species as an alternative transmission vector. The introduced parasite may negatively influence native host abundance, thus influencing invasion success of its co-introduced host, as shown here. This may in turn lead to greater infection levels in definitive hosts in the recipient ecosystem than in the original ecosystem of the parasite (situation not represented here) (C) The non-native parasite is introduced without its original host (or this host does not survive translocation) but is subsequently included in the recipient food web. The novel parasite may in turn have drastic effects on intermediate and/or native hosts and reach higher infection levels in these novel hosts as represented here. However, a multitude of alternative scenarios are possible with as many outcomes in terms of parasite dynamics.
Parasites are generally more likely to transfer to native hosts phylogenetically close to their original host (Strauss et al., 2012). Introduced parasites may thus infect congeneric native host species through spillover from their original, co-introduced host (Daszak et al., 2000). For parasites with complex life cycles such as acanthocephalans or trematodes, survival and establishment depends on the presence of a combination of multiple, phylogenetically distant suitable hosts (Blakeslee and Fowler, 2012). For example, trematodes are highly specific to their snail first intermediate hosts but are somewhat more flexible regarding second intermediate and definitive hosts (Graczyk, 1997). Availability of suitable native hosts to introduced parasites may strongly influence the presence or absence of such parasites. As a result, species with complex life cycles often retain one of their original, co-introduced hosts and use novel, native hosts to fill in the gaps and complete their life cycle (Taraschewski, 2006, Fig. 3A). In extreme cases, introduced parasites may even invade new host communities without the introduction of the original host(s) (Fig. 3C). The nematode parasite Anguillicola crassus is native to Japan where it infects the eel Anguilla japonica but is now found in the European eel Anguilla anguilla (Starkie, 2003, Norton et al., 2005). Introduction of Anguillicola crassus in Europe was partly through shipments of infected A. Japonica but also, in some places, water containing only eggs of the parasite, not infected hosts (Kennedy and Fitch, 1990, Kirk, 2003). The life cycle was then completed through native crustacean intermediate hosts and A. anguilla (Kirk, 2003). Generally, invasive parasites are co-introduced with one of their original, invasive host species and subsequently infect native hosts. Of the very few studies that looked at introduced parasite virulence on both co-introduced and native hosts, 85% show that virulence was higher in native hosts (Lymbery et al., 2014). These parasites may subsequently have catastrophic effects on native host populations through increased host mortality and decreased fecundity rates (McCallum and Dobson, 1995, Hudson et al., 1998, Ieshko et al., 2013, Fig. 3B). Transfer to new, local hosts, coupled with potentially increased virulence, produces a complex combination of factors with potentially large impacts on introduced parasites (see Fig. 3 for some potential scenarios). However, the overall results on parasite population dynamics, compared to that observed in the original hosts are hardly ever assessed. Furthermore, successful invasions and parasite transfer to new hosts resulting in high infection levels or pathogenic impacts tend to be documented and attract scientific attention but represent only part of the picture (Kennedy, 1993). Negative changes in parasite dynamics following invasion are seldom documented but represent perfect opportunities to try and understand why some introduced parasite transfer are successful and other fail.
5.3. Native hosts and introduced parasites
Introduced host species often lose most of their parasites during invasion. However, the few co-introduced parasites that do stow-away may spillover to native hosts with potentially considerable impacts on these naive hosts (Mastitsky et al., 2014, Fig. 3). One well-known example is the directly transmitted oomycete pathogen Aphanomyces astaci causing crayfish plague. The oomycete was transmitted from invasive North American crayfish to native crayfish in large parts of Europe, resulting in a rapid spread of the disease followed by the decline of many populations of native crayfish (Holdich and Reeve, 1991, Holdich et al., 2009). In some cases, it was also followed by local extinction of the pathogen due to a subsequent lack of available hosts (Reynolds, 1988). Although the effects of crayfish plague on native crayfish host population dynamics have been extensively studied, the pathogen itself and its own dynamics have never been the focus of any studies. Similarly, the cestode Ligula intestinalis has been the focus of many studies for its pathological effects on fish hosts in both the native and invasive range of the parasite (Carter et al., 2005, Cowx et al., 2008, Hoole et al., 2010). Parasite dynamics in fish hosts have been well-studied too (Kennedy and Burrough, 1981, Kennedy et al., 2001). However, L. intestinalis has a three host life cycle also including a copepod crustacean (Dubinina, 1966). In areas where the parasite has been introduced, mostly with infected fish or bird hosts, the life cycle is completed using native copepod species but infection levels and potential effects on native copepod host populations have never been documented and/or compared to that observed in native copepods. In New Zealand, where the parasite was likely introduced by Australasian crested grebes (Podiceps cristatus australis) migrating from Australia, the copepod host has not even been identified yet (Weekes and Penlington, 1986, Lagrue et al., 2017).
Parasites introduced without their original hosts can also colonize novel hosts with the same dramatic consequences on native, novel hosts as Aphanomyces astaci on European crayfish species (see above). The eel nematode Anguillicola crassus has little effect in its original host Anguilla japonica but can have severe pathological impacts on the highly susceptible European eel A. anguilla within which it reaches higher parasite burdens than in A. japonica (Kirk, 2003). The parasites have been shown to have severe impacts on their novel eel host, both at the individual and population level; heavily infected eels showing increased mortality rates and being potentially unable to migrate and spawn (Kirk, 2003, Fig. 3C). Invasion of the Asian isopod parasite Orthione griffenis in North America coincided with the collapse of populations of the native mud shrimp Upogebia pugettensis; infection by the introduced parasite caused severe reduction in fecundity in the naïve native host with potentially dramatic effects on the whole ecosystem (Dumbauld et al., 2011). Similarly, on the Atlantic coast of America, native mud crabs Eurypanopeus depressus are infected by the invasive barnacle parasite (Loxothylacus panopaei) that castrates its host and renders it more prone to consumption by native predators (Gehman and Byers, 2017). The preferential consumption of native prey infected by introduced parasites by native predators may alter predator-prey dynamics and have in depth effects on the whole food chain and affect parasite infection levels and ultimately its ability to persist in this novel host (Gehman and Byers, 2017). The combined implications of higher host susceptibility to infection, increased parasite burdens but also host mortality or lower fecundity on the population dynamics of the parasite itself are unclear, however.
Both the nematode A. crassus and cestode L. intestinalis use native copepod hosts for transmission to the next host but parasite dynamics in their novel, native crustacean hosts are unknown. Furthermore, introduced parasites may in turn impact their novel, native hosts and thus have complex cascading effects on recipient communities and native parasite dynamics (Médoc et al., 2017). Many known examples of introduced parasites are freshwater species, reflecting the vulnerability of aquatic ecosystems to invasion by new pathogens (Lymbery et al., 2014, Médoc et al., 2017). Well-documented examples of spillover of invasive parasites in native crustacean hosts are scant however, let alone quantitative assessment of parasite dynamics.
6. Conclusion
Aquatic ecosystems in general, and fresh waters in particular, are among the most invaded in the world and have been the focus of multiple studies contributing to our knowledge of the mechanisms influencing invasion success and the potential effects of introduced species (Ricciardi and MacIsaac, 2011). There is also increasing evidence that parasites play important roles in biological invasions. However, the impacts of biological invasion on parasite dynamics have clearly received less attention than the potential role of pathogens in invasion processes, especially in aquatic ecosystems even though freshwater parasites can be affected by introduced hosts (Taraschewski, 2006). For example, several studies have shown that invasive fish species can have drastic effects on local parasite communities and infection levels in native host species (Font and Tate, 1994, Dubois et al., 1996, Bergeron et al., 1997, Amundsen et al., 2013, Ieshko et al., 2013). However, studies focusing on the effects of biological invasions on aquatic parasite dynamics are still relatively rare (Blakeslee and Fowler, 2012). Furthermore, these studies most often focus on vertebrate definitive hosts of parasites, especially fish species as shown above (Poulin et al., 2011, Blakeslee and Fowler, 2012). Invertebrate species in general and crustaceans in particular are hosts to a multitude of parasites and are some of the most common invasive species in aquatic ecosystems (Hatcher et al., 2015). Yet, their potential effects on parasite dynamics following invasion of new ecosystems are very rarely accounted for. This partly comes from the logistical difficulties of such studies and the recurrent lack of reference points to which we can compare observed post-invasion patterns (Blakeslee and Fowler, 2012). Patterns of parasite communities and infection levels post-invasion can be extremely complex and give no information on changes in parasite dynamics specifically due to biological invasion (Grabner et al., 2015). An obvious way to assess biological invasion impacts on parasite dynamics would be to examine native disease dynamics in communities before and after invasion in natural settings (Telfer et al., 2005). When invasion fronts are known and identified, it should be possible to document parasite dynamics, along with host diversity and density, ahead of and behind the front of invasion. Alternatively, because aquatic systems, especially freshwater ecosystems, are patchy by nature, some invasive species are also patchily distributed, providing spatial replicates allowing a comparison of parasite population dynamics with and without invaders; see Dubois et al. (1996) and Bergeron et al. (1997) for excellent examples of study designs making great use of the patchy nature of freshwater systems and invasive fish species distributions. These studies highlight the complex and dramatic consequences of non-native fish introduction on parasite communities of both native and introduced fish host species in the field by using comparable lake ecosystems with and without introduced fish as geographical replicates. Unfortunately, these studies have been largely ignored and should be acknowledged for being some of the first to quantify the effects of invasive species on both native and co-introduced parasite communities. Observed field patterns should then be used in theoretical and empirical studies to try and elucidate key processes influencing disease dynamics following biological invasions (Telfer and Bown, 2012). There is a clear need for well-designed mesocosm and field experiments comparing parasite dynamics in the presence or absence of invasive crustaceans. Ideally and eventually, general predictions of how these commonly invasive taxa may influence disease dynamics may be drawn.
Acknowledgements
I am grateful to Robert Poulin and Bronwen Presswell, Andrew Thompson and an anonymous reviewer for very constructive comments on a previous version of the manuscript. CL is funded by a grant from the Royal Society of New Zealand's Marsden Fund (UOO1408) (salary).
References
- Amundsen P.A., Lafferty K.D., Knudsen R., Primicerio R., Kristoffersen R., Klemetsen A., Kuris A.M. New parasites and predators follow the introduction of two fish species to a subarctic lake: implications for food-web structure and functioning. Oecologia. 2013;171:993–1002. doi: 10.1007/s00442-012-2461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundell K., Dunn A., Alexander J., Shearman R., Archer N., Ironside J.E. Enemy release and genetic founder effects in invasive killer shrimp populations of Great Britain. Biol. Invasions. 2015;17:1439–1451. [Google Scholar]
- Bacela-Spychalska K., Wattier R.A., Genton C., Rigaud T. Microsporidian disease of the invasive amphipod Dikerogammarus villosus and the potential for its transfer to local invertebrate fauna. Biol. Invasions. 2012;14:1831–1842. [Google Scholar]
- Bauer A., Rigaud T. Identifying a key host in an acanthocephalan-amphipod system. Parasitology. 2015;142:1588–1594. doi: 10.1017/S0031182015001067. [DOI] [PubMed] [Google Scholar]
- Bauer A., Trouvé S., Grégoire A., Bollache L., Cézilly F. Differential influence of Pomphorhynchus laevis (acanthocephala) on the behaviour of native and invader gammarid species. Int. J. Parasitol. 2000;30:1453–1457. doi: 10.1016/s0020-7519(00)00138-7. [DOI] [PubMed] [Google Scholar]
- Bergeron M., Marcogliese D.J., Magnan P. The parasite fauna of brook trout, Salvelinus fontinalis (Mitchill), in relation to lake morphometrics and the introduction of creek chub, Semotilus atromaculatus (Mitchill) Ecoscience. 1997;4:427–436. [Google Scholar]
- Bij de Vaate A., Jazdzewski K., Ketelaars H.A., Gollasch S., Van der Velde G. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can. J. Fish. Aquat. Sci. 2002;59:1159–1174. [Google Scholar]
- Blackburn T.M., Ewen J.G. Parasites as drivers and passengers of human-mediated biological invasions. EcoHealth. 2016:1–13. doi: 10.1007/s10393-015-1092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee A.M., Altman I., Miller A.W., Byers J.E., Hamer C.E., Ruiz G.M. Parasites and invasions: a biogeographic examination of parasites and hosts in native and introduced ranges. J. Biogeogr. 2012;39:609–622. [Google Scholar]
- Blakeslee A.M.H., Fowler A.E. Aquatic introductions and genetic founder effects: how do parasites compare to hosts? In: Caliskan M., editor. Analysis of Genetic Variation in Animals. InTech Open Access Publisher; 2012. pp. 315–336. [Google Scholar]
- Blakeslee A.M.H., Keogh C.L., Byers J.E., Kuris A.M., Lafferty K.D., Torchin M.E. Differential escape from parasites by two competing introduced crabs. Mar. Ecol. Prog. Ser. 2009;393:83–96. [Google Scholar]
- Blakeslee A.M.H., Keogh C.L., Fowler A.E., Griffen B.D. Assessing the effects of trematode infection on invasive green crabs in Eastern North America. PloS One. 2015;10:e0128674. doi: 10.1371/journal.pone.0128674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollache L., Devin S., Wattier R.A., Chovet M., Beisel J.N., Moreteau J.C., Rigaud T. Rapid range extension of the Ponto-Caspian amphipod Dikerogammarus villosus in France: potential consequences. Arch. für Hydrobiol. 2004;160:57–66. [Google Scholar]
- Bollache L., Kaldonski N., Troussard J.P., Lagrue C., Rigaud T. Spines and behaviour as defences against fish predators in an invasive freshwater amphipod. Anim. Behav. 2006;72:627–633. [Google Scholar]
- Britton J.R. Introduced parasites in food webs: new species, shifting structures? Trends Ecol. Evol. 2013;28:93–99. doi: 10.1016/j.tree.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Carter V., Pierce R., Dufour S., Arme C., Hoole D. The tapeworm Ligula intestinalis (Cestoda: Pseudophyllidea) inhibits LH expression and puberty in its teleost host, Rutilus rutilus. Reproduction. 2005;130:939–945. doi: 10.1530/rep.1.00742. [DOI] [PubMed] [Google Scholar]
- Cézilly F., Grégoire A., Bertin A. Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology. 2000;120:625–630. doi: 10.1017/s0031182099005910. [DOI] [PubMed] [Google Scholar]
- Chovet M., Lécureuil J. Répartition des Gammaridae épigés (Crustacés, Amphipodes) dans la Loire et les rivières de la Région Centre (France) Ann. Limnol. 1994;30:11–23. [Google Scholar]
- Clay K. Parasites lost. Nature. 2003;421:585–586. doi: 10.1038/421585a. [DOI] [PubMed] [Google Scholar]
- Cornet S., Franceschi N., Bauer A., Rigaud T., Moret Y. Immune depression induced by acanthocephalan parasites in their intermediate crustacean host: consequences for the risk of super-infection and links with host behavioural manipulation. Int. J. Parasitol. 2009;39:221–229. doi: 10.1016/j.ijpara.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Cornet S., Sorci G., Moret Y. Biological invasion and parasitism: invaders do not suffer from physiological alterations of the acanthocephalan Pomphorhynchus laevis. Parasitology. 2010;137:137–147. doi: 10.1017/S0031182009991077. [DOI] [PubMed] [Google Scholar]
- Cowx I.G., Rollins D., Tumwebaze R. Effect of Ligula intestinalis on the reproductive capacity of Rastrineobola argentea in Lake Victoria. J. Fish Biol. 2008;73:2249–2260. [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Devin S., Bollache L., Noël P.Y., Beisel J.N. Patterns of biological invasions in French freshwater systems by non-indigenous macroinvertebrates. Hydrobiologia. 2005;551:137–146. [Google Scholar]
- Dick J.T.A., Platvoet D. Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc. R. Soc. Lond. B. 2000;267:977–983. doi: 10.1098/rspb.2000.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J.M. The paradox of the parasites: implications for biological invasion. Proc. R. Soc. Lond. B. 2003;270:S133–S135. doi: 10.1098/rsbl.2003.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinina M.N. Amerind Publishing Co.; New Delhi: 1966. Tapeworms (Cestoda, Ligulidae) of the Fauna of the USSR. [Google Scholar]
- Dubois N., Magnan P., Marcogliese D.J. Effects of the introduction of white sucker, Catostomus commersoni, on the parasite fauna of brook trout, Salvelinus fontinalis. Can. J. Zool. 1996;74:1304–1312. [Google Scholar]
- Dumbauld B.R., Chapman J.W., Torchin M.E., Kuris A.M. Is the collapse of mud shrimp (Upogebia pugettensis) populations along the Pacific coast of North America caused by outbreaks of a previously unknown bopyrid isopod parasite (Orthione griffenis)? Estuaries Coasts. 2011;34:336–350. [Google Scholar]
- Dunn A.M. Parasites and biological invasions. Adv. Parasitol. 2009;68:161–184. doi: 10.1016/S0065-308X(08)00607-6. [DOI] [PubMed] [Google Scholar]
- Dunn A.M., Dick J.T.A. Parasitism and epibiosis in native and non-native gammarids in freshwater in Ireland. Ecography. 1998;21:593–598. [Google Scholar]
- Dunn A.M., Hatcher M.J. Parasites and biological invasions: parallels, interactions, and control. Trends Parasitol. 2015;31:189–199. doi: 10.1016/j.pt.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Dunn A.M., Perkins S.E. Editorial: invasions and infections. Funct. Ecol. 2012;26:1234–1237. [Google Scholar]
- Dunn A.M., Torchin M.E., Hatcher M.J., Kotanen P.M., Blumenthal D.M., Byers J.E., Coon C.A.C., Frankel V.M., Holt R.D., Hufbauer R.A., Kanarek A.R., Schierenbeck K.A., Wolfe L.M., Perkins S.E. Indirect effects of parasites in invasions. Funct. Ecol. 2012;26:1262–1274. [Google Scholar]
- Ebert D. Virulence and local adaptation of a horizontally transmitted parasite. Science. 1994;265:1084–1086. doi: 10.1126/science.265.5175.1084. [DOI] [PubMed] [Google Scholar]
- Emblidge Fromme A., Dybdahl M.F. Resistance in introduced populations of a freshwater snail to native range parasites. J. Evol. Biol. 2006;19:1948–1955. doi: 10.1111/j.1420-9101.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- Emde S., Rueckert S., Palm H.W., Klimpel S. Invasive Ponto-Caspian amphipods and fish increase the distribution range of the acanthocephalan Pomphorhynchus tereticollis in the river Rhine. Plos One. 2012;7:e53218. doi: 10.1371/journal.pone.0053218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmat C., Alibert P., Mutin G., Losseau M., Pariselle A., Sasal P. A case of complete loss of gill parasites in the invasive cichlid Oreochromis mossambicus. Parasitol. Res. 2016;115:3657–3661. doi: 10.1007/s00436-016-5168-1. [DOI] [PubMed] [Google Scholar]
- Font W.F., Tate D.C. Helminth parasites of native Hawaiian freshwater fishes: an example of extreme ecological isolation. J. Parasitol. 1994;80:682–688. [PubMed] [Google Scholar]
- Gehman A.L.M., Byers J.E. Non-native parasite enhances susceptibility of host to native predators. Oecologia. 2017;183:919–926. doi: 10.1007/s00442-016-3784-1. [DOI] [PubMed] [Google Scholar]
- Gendron A.D., Marcogliese D.J. Reduced survival of a native parasite in the invasive round goby: evidence for the dilution hypothesis? Aquat. Invasions. 2016;11:189–198. [Google Scholar]
- Gendron A.D., Marcogliese D.J., Thomas M. Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St. Lawrence Basin. Biol. Invasions. 2012;14:367–384. [Google Scholar]
- Genner M.J., Michel E., Todd J.A. Resistance of an invasive gastropod to an indigenous trematode parasite in Lake Malawi. Biol. Invasions. 2008;10:41–49. [Google Scholar]
- Georgiev B.B., Sánchez M.I., Vasileva G.P., Nikolov P.N., Green A.J. Cestode parasitism in invasive and native brine shrimps (Artemia spp.) as a possible factor promoting the rapid invasion of A. franciscana in the Mediterranean region. Parasitol. Res. 2007;101:1647–1655. doi: 10.1007/s00436-007-0708-3. [DOI] [PubMed] [Google Scholar]
- Gherardi F. Biological invasions in inland waters: an overview. In: Gherardi F., editor. Biological Invaders in Inland Waters: Profiles, Distribution, and Threats. Springer; Dordrecht: 2007. pp. 3–25. [Google Scholar]
- Gherardi F., Gollasch S., Minchin D., Olenin S., Panov V.E. Alien invertebrates and fish in European inland waters. In: Hulme P., Netwig W., Pysek P., Vilà M., editors. Handbook of Alien Species in Europe. Springer; Dordrecht: 2009. pp. 81–92. [Google Scholar]
- Grabner D.S. Hidden diversity: parasites of stream arthropods. Freshw. Biol. 2017;62:52–64. [Google Scholar]
- Grabner D.S., Weigand A.M., Leese F., Winking C., Hering D., Tollrian R., Sures B. Invaders, natives and their enemies: distribution patterns of amphipods and their microsporidian parasites in the Ruhr Metropolis, Germany. Parasites Vectors. 2015;8:419. doi: 10.1186/s13071-015-1036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski M., Bacela K., Konopacka A. How to be an invasive gammarid: comparison of life history traits. Hydrobiologia. 2007;590:75–84. [Google Scholar]
- Graczyk T.K. Immunobiology of trematodes in vertebrate hosts. In: Fried B., Graczyk T.K., editors. Advances in Trematode Biology. CRC Press; Boca Raton, FL: 1997. pp. 384–398. [Google Scholar]
- Guégan J.F., Kennedy C.R. Maximum local helminth parasite community richness in British freshwater fish: a test of the colonization time hypothesis. Parasitology. 1993;106:91–100. doi: 10.1017/s0031182000074862. [DOI] [PubMed] [Google Scholar]
- Haine E.R., Motreuil S., Rigaud T. Infection by a vertically transmitted microsporidian parasite is associated with a female-biased sex ratio and survival advantage in the amphipod Gammarus roeseli. Parasitology. 2007;134:1363–1367. doi: 10.1017/S0031182007002715. [DOI] [PubMed] [Google Scholar]
- Hall S., Becker C., Simonis J., Duffy M. Friendly competition: evidence for a dilution effect among competitors in a planktonic host-parasite system. Ecology. 2009;90:791–801. doi: 10.1890/08-0838.1. [DOI] [PubMed] [Google Scholar]
- Hänfling B., Edwards F., Gherardi F. dispersal, establishment, impact and control. BioControl. 2011;56:573–595. Invasive alien Crustacea. [Google Scholar]
- Hatcher M.J., Dick J.T.A., Dunn A.M. How parasites affect interactions between competitors and predators. Ecol. Lett. 2006;9:1253–1271. doi: 10.1111/j.1461-0248.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- Hatcher M.J., Dick J.T.A., Dunn A.M. Disease emergence and invasions. Funct. Ecol. 2012;26:1275–1287. doi: 10.1111/j.1365-2435.2012.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher M.J., Dick J.T.A., Dunn A.M. Diverse effects of parasites in ecosystems: linking interdependent processes. Front. Ecol. Environ. 2012;10:186–194. [Google Scholar]
- Hatcher M.J., Dick J.T.A., Paterson R.A., Alexander M.E., Bunke M., Dunn A.M. Trait-mediated effects of parasites on invader-native interactions. In: Mehlhorn H., editor. Host Manipulations by Parasites and Viruses. Springer; Dordrecht: 2015. pp. 29–47. [Google Scholar]
- Hatcher M.J., Dunn A.M. Cambridge University Press; Cambridge: 2011. Parasites in Ecological Communities: from Interactions to Ecosystems. Ecology, Biodiversity and Conservation. [Google Scholar]
- Holdich D.M., Reeve I.D. Distribution of freshwater crayfish in the British Isles, with particular reference to crayfish plague, alien introductions and water quality. Aquat. Conserv. Mar. Freshw. Ecosyst. 1991;1:139–158. [Google Scholar]
- Holdich D.M., Reynolds J.D., Souty-Grosset C., Sibley P.J. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst. 2009;394–395:11. [Google Scholar]
- Hoole D., Carter V., Dufour S. Ligula intestinalis (Cestoda: Pseudophyllidea): an ideal fish-metazoan parasite model? Parasitology. 2010;137:425–438. doi: 10.1017/S0031182010000107. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Lafferty K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- Ieshko E.P., Shul’man B.S., Lebedeva D.I., Barskaya Y.Y., Niemela E. Bullhead (Cottus gobio L.) invasion in the Utsjoki River (Northern Finland): Parasitological aspects. Russ. J. Biol. Iinvasions. 2013;4:17–23. [Google Scholar]
- Jackson C.J., Marcogliese D.J., Burt M.D. Role of hyperbenthic crustaceans in the transmission of marine helminth parasites. Can. J. Fish. Aquat. Sci. 1997;54:815–820. [Google Scholar]
- Johnson P.T.J., Lund P.J., Hartson R.B., Yoshino T.P. Community diversity reduces Schistosoma mansoni transmission, host pathology and human infection risk. Proc. R. Soc. Lond. B. 2009;276:1657–1663. doi: 10.1098/rspb.2008.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldonski N., Lagrue C., Motreuil S., Rigaud T., Bollache L. Habitat segregation mediates predation by the benthic fish Cottus gobio on the exotic amphipod species Gammarus roeseli. Naturwissenschaften. 2008;95:839–844. doi: 10.1007/s00114-008-0392-x. [DOI] [PubMed] [Google Scholar]
- Kaldonski N., Perrot-Minnot M.J., Cézilly F. Differential influence of two acanthocephalan parasites on the antipredator behaviour of their common intermediate host. Anim. Behav. 2007;74:1311–1317. [Google Scholar]
- Kaltz O., Shykoff J. Local adaptation in host-parasite systems. Heredity. 1998;81:361–370. [Google Scholar]
- Karatayev A.Y., Burlakova L.E., Padilla D.K., Mastitsky S.E., Olenin S. Invaders are not a random selection of species. Biol. Invasions. 2009;11:2009–2019. [Google Scholar]
- Keane R.M., Crawley M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002;17:164–170. [Google Scholar]
- Keller R.P., Geist J., Jeschke J.M., Kühn I. Invasive species in Europe: ecology, status, and policy. Environ. Sci. Eur. 2011;23:23. [Google Scholar]
- Kelly D.W., Paterson R.A., Townsend C.R., Poulin R., Tompkins D.M. Has the introduction of brown trout altered disease patterns in native New Zealand fish? Freshw. Biol. 2009;54:1805–1818. [Google Scholar]
- Kelly D.W., Paterson R.A., Townsend C.R., Poulin R., Tompkins D.M. Parasite spillback: a neglected concept in invasion ecology? Ecology. 2009;90:2047–2056. doi: 10.1890/08-1085.1. [DOI] [PubMed] [Google Scholar]
- Kennedy C.R. Introductions, spread and colonization of new localities by fish helminth and crustacean parasites in the British Isles: a perspective and appraisal. J. Fish Biol. 1993;43:287–301. [Google Scholar]
- Kennedy C.R. Cambridge University Press; Cambridge, UK: 2006. Ecology of the Acanthocephala. [Google Scholar]
- Kennedy C.R., Burrough R.J. The establishment and subsequent history of a population of Ligula intestinalis in roach Rutilus rutilus (L.) J. Fish Biol. 1981;19:105–126. [Google Scholar]
- Kennedy C.R., Fitch D.J. Colonization, larval survival and epidemiology of the nematode Anguillicola crassus, parasitic in the eel, Anguilla anguilla, in Britain. J. Fish Biol. 1990;36:117–131. [Google Scholar]
- Kennedy C.R., Shears P.C., Shears J.A. Long-term dynamics of Ligula intestinalis and roach Rutilus rutilus: a study of three epizootic cycles over thirty-one years. Parasitology. 2001;123:257–269. doi: 10.1017/s0031182001008538. [DOI] [PubMed] [Google Scholar]
- Kirk R.S. The impact of Anguillicola crassus on European eels. Fish. Manag. Ecol. 2003;10:385–394. [Google Scholar]
- Knudsen R. Relationships between habitat, prey selection and parasite infection in Arctic charr (Salvelinus alpinus) Nord. J. Freshw. Res. 1995;71:333–344. [Google Scholar]
- Kopp K., Jokela J. Resistant invaders can convey benefits to native species. Oikos. 2007;116:295–301. [Google Scholar]
- Krakau M., Thieltges D.W., Reise K. Native parasites adopt introduced bivalves of the North Sea. Biol. Invasions. 2006;8:919–925. [Google Scholar]
- Kuris A.M., Hechinger R.F., Shaw J.C., Whitney K.L., Aguirre-Macedo L., Boch C.A., Dobson A.P., Dunham E.J., Fredensborg B.L., Huspeni T.C., Lorda J., Mababa L., Mancini F.T., Mora A.B., Pickering M., Talhouk N.L., Torchin M.E., Lafferty K.D. Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature. 2008;454:515–518. doi: 10.1038/nature06970. [DOI] [PubMed] [Google Scholar]
- Kuris A.M., Lafferty K.D. Modelling crustacean fisheries: effects of parasites on management strategies. Can. J. Fish. Aquat. Sci. 1992;49:327–336. [Google Scholar]
- Lafferty K.D., Dobson A.P., Kuris A.M. Parasites dominate food web links. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D., Kuris A.M. Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol. 2009;25:564–572. doi: 10.1016/j.pt.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lagrue C., Kaldonski N., Perrot-Minnot M.J., Motreuil S., Bollache L. Modification of host's behavior by a parasite: field evidence for adaptive manipulation. Ecology. 2007;88:2839–2847. doi: 10.1890/06-2105.1. [DOI] [PubMed] [Google Scholar]
- Lagrue C., Poulin R. The scaling of parasite biomass with host biomass in lake ecosystems: are parasites limited by host resources? Ecography. 2016;39:507–514. [Google Scholar]
- Lagrue C., Presswell B., Dunckley N., Poulin P. The invasive cestode parasite Ligula from salmonids and bullies in Lake Hawea, South Island. N. Z. J. Mar. Freshw. Res. 2017 doi: 10.1007/s00436-017-5684-7. (Under review) [DOI] [PubMed] [Google Scholar]
- Laverty C., Nentwig W., Dick J.T.A., Lucy F.E. Alien aquatics in Europe: assessing the relative environmental and socioeconomic impacts of invasive aquatic macroinvertebrates and other taxa. Manag. Biol. Invasions. 2015;6:341–350. [Google Scholar]
- Lettoof D.C., Greenlees M.J., Stockwell M., Shine R. Do invasive cane toads affect the parasite burdens of native Australian frogs? Int. J. Parasitol. Parasites Wildl. 2013;2:155–164. doi: 10.1016/j.ijppaw.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry E., Rollinson E.J., Laybourn A.J., Scott T.E., Aiello-Lammens M.E., Gray S.M., Mickley J., Gurevitch J. Biological invasions: a field synopsis, systematic review, and database of the literature. Ecol. Evol. 2013;3:182–196. doi: 10.1002/ece3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymbery A.J., Morine M., Kanani H.G., Beatty S.J., Morgan D.L. Co-invaders: the effects of alien parasites on native hosts. Int. J. Parasitol. Parasites Wildl. 2014;3:171–177. doi: 10.1016/j.ijppaw.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil C., Dick J.T. Parasite-mediated intraguild predation as one of the drivers of co-existence and exclusion among invasive and native amphipods (Crustacea) Hydrobiologia. 2011;665:247–256. [Google Scholar]
- MacNeil C., Dick J.T.A., Hatcher M.J., Dunn A.M. Differential drift and parasitism in invading and native Gammarus spp. (Crustacea: Amphipoda) Ecography. 2003;26:467–473. [Google Scholar]
- Macneil C., Fielding N.J., Dick J.T.A., Briffa M., Prenter J., Hatcher M.J., Dunn A.M. An acanthocephalan parasite mediates intraguild predation between invasive and native freshwater amphipods (Crustacea) Freshw. Biol. 2003;48:2085–2093. [Google Scholar]
- MacNeil C., Fielding N.J., Hume K.D., Dick J.T.A., Elwood R.W., Hatcher M.J., Dunn A.M. Parasite altered microdistribution of Gammarus pulex (Crustacea: Amphipoda) Int. J. Parasitol. 2003;33:57–64. doi: 10.1016/s0020-7519(02)00229-1. [DOI] [PubMed] [Google Scholar]
- Marcogliese D.J. The role of zooplankton in the transmission of helminth parasites to fish. Rev. Fish Biol. Fish. 1995;5:336–371. [Google Scholar]
- Marcogliese D.J. Parasites of the superorganism: are they indicators of ecosystem health? Int. J. Parasitol. 2005;35:705–716. doi: 10.1016/j.ijpara.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Marcogliese D.J., Cone D.K. Food webs: a plea for parasites. Trends Ecol. Evol. 1997;12:320–325. doi: 10.1016/S0169-5347(97)01080-X. [DOI] [PubMed] [Google Scholar]
- Mastitsky S.E., Karatayev A.Y., Burlakova L.E., Molloy D.P. Parasites of exotic species in invaded areas: does lower diversity mean lower epizootic impact? Divers. Distrib. 2010;16:798–803. [Google Scholar]
- Mastitsky S.E., Karatayev A.Y., Burlakova L.E. Parasites of aquatic exotic invertebrates: identification of potential risks posed to the Great Lakes. Hum. Ecol. Risk Assess. An Int. J. 2014;20:743–763. [Google Scholar]
- McCallum H., Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- Médoc V., Bollache L., Beisel J.N. Host manipulation of a freshwater crustacean (Gammarus roeseli) by an acanthocephalan parasite (Polymorphus minutus) in a biological invasion context. Int. J. Parasitol. 2006;36:1351–1358. doi: 10.1016/j.ijpara.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Médoc V., Firmat C., Sheath D.J., Pegg J., Andreou D., Britton J.R. Parasites and biological invasions: predicting ecological alterations at levels from individual hosts to whole networks. Adv. Ecol. Res. 2017 [Google Scholar]
- Miller A.W., Ruiz G.M. Differentiating successful and failed invaders: species pools and the importance of defining vector, source and recipient regions. In: Rilov G., Crooks J., editors. Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives. Springer Verlag; Berlin: 2009. pp. 153–170. [Google Scholar]
- Milner R.J., Mayer J.A. Tuzetia boeckella sp. nov. (Protozoa: Microsporida), a parasite of Boeckella triarticulata (Copepoda: Calanoidea) in Australia. J. Invertebr. Pathol. 1982;39:174–184. [Google Scholar]
- Moret Y., Bollache L., Wattier R., Rigaud T. Is the host or the parasite the most locally adapted in an amphipod–acanthocephalan relationship? A case study in a biological invasion context. Int. J. Parasitol. 2007;37:637–644. doi: 10.1016/j.ijpara.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Mouritsen K.N., Poulin R. Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology. 2002;124:S101–S117. doi: 10.1017/s0031182002001476. [DOI] [PubMed] [Google Scholar]
- Nalepa T.F., Fanslow D.L., Lang G.A. Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to the invasive mussel Dreissena rostriformis bugensis. Freshw. Biol. 2009;54:466–479. [Google Scholar]
- Norton J., Rollinson D., Lewis J.W. Epidemiology of Anguillicola crassus in the European eel (Anguilla anguilla) from two rivers in southern England. Parasitology. 2005;130:679–686. doi: 10.1017/s0031182004007139. [DOI] [PubMed] [Google Scholar]
- Ostfeld R.S., Keesing F. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst. 2012;43:157–182. [Google Scholar]
- Ovcharenko M., Bacela K., Wilkinson T., Ironside J., Rigaud T., Wattier R.A. Cucumispora dikerogammari n. gen. (Fungi: Microsporidia) infecting the invasive amphipod Dikerogammarus villosus: a potential emerging disease in European rivers. Parasitology. 2010;137:191–204. doi: 10.1017/S0031182009991119. [DOI] [PubMed] [Google Scholar]
- Paterson R.A., Lal A., Dale M., Townsend C.R., Poulin R., Tompkins D.M. Relative competence of native and exotic fish hosts for two generalist native trematodes. Int. J. Parasitol. Parasites Wildl. 2013;2:136–143. doi: 10.1016/j.ijppaw.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.A., Rauque C.A., Fernandez M.V., Townsend C.R., Poulin R., Tompkins D.M. Native fish avoid parasite spillback from multiple exotic hosts: consequences of host density and parasite competency. Biol. Invasions. 2013;15:2205–2218. [Google Scholar]
- Paterson R.A., Townsend C.R., Poulin R., Tompkins D.M. Introduced brown trout alter native acanthocephalan infections in native fish. J. Anim. Ecol. 2011;80:990–998. doi: 10.1111/j.1365-2656.2011.01834.x. [DOI] [PubMed] [Google Scholar]
- Peeler E.J., Oidtmann B.C., Midtlyng P.J., Miossec L., Gozlan R.E. Non-native aquatic animal introductions have driven disease emergence in Europe. Biol. Invasions. 2011;13:1291–1303. [Google Scholar]
- Poulin R., Paterson R.A., Townsend C.R., Tompkins D.M., Kelly D.W. Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshw. Biol. 2011;56:676–688. [Google Scholar]
- Prenter J., Macneil C., Dick J.T.A., Dunn A.M. Roles of parasites in animal invasions. Trends Ecol. Evol. 2004;19:385–390. doi: 10.1016/j.tree.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Price P.W., Westoby M., Rice B., Atsatt P.R., Fritz R.S., Thompson J.N., Mobley K. Parasite mediation in ecological interactions. Annu. Rev. Ecol. Syst. 1986;17:487–505. [Google Scholar]
- Pulkkinen K., Ruokonen T.J., Mykrä M., Tambe G., Karjalainen J., Hämäläinen H. Indirect effects of invasive crayfish on native fish parasites. Ecosphere. 2013;4:1–9. [Google Scholar]
- Redón S., Amat F., Sánchez M.I., Green A.J. Comparing cestode infections and their consequences for host fitness in two sexual branchiopods: alien Artemia franciscana and native A. salina from syntopic-populations. PeerJ. 2015;3:e1073. doi: 10.7717/peerj.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard E.G. Parasitic castration of crustacea. Exp. Parasitol. 1956;5:79–107. doi: 10.1016/0014-4894(56)90007-8. [DOI] [PubMed] [Google Scholar]
- Reisinger L.S., Lodge D.M. Parasites alter freshwater communities in mesocosms by modifying invasive crayfish behavior. Ecology. 2016;97:1497–1506. doi: 10.1890/15-1634.1. [DOI] [PubMed] [Google Scholar]
- Reynolds J.D. Crayfish extinctions and crayfish plague in central Ireland. Biol. Conserv. 1988;45:279–285. [Google Scholar]
- Ricciardi A. Ecology of invasive alien invertebrates. In: Roger D.C., editor. Ecology and General Biology: Thorp and Covich's Freshwater Invertebrates. Academic Press; Cambridge: 2015. pp. 83–91. [Google Scholar]
- Ricciardi A., MacIsaac H.J. Recent mass invasion of the North American Great Lakes by Ponto-Caspian species. Trends Ecol. Evol. 2000;15:62–65. doi: 10.1016/s0169-5347(99)01745-0. [DOI] [PubMed] [Google Scholar]
- Ricciardi A., MacIsaac H.J. Impacts of biological invasions on freshwater ecosystems. In: Richardson D.M., editor. Fifty Years of Invasion Ecology: the Legacy of Charles Elton. Wiley-Blackwell; Hoboken: 2011. pp. 211–224. [Google Scholar]
- Rigaud T., Moret Y. Differential phenoloxidase activity between native and invasive gammarids infected by local acanthocephalans: differential immunosuppression? Parasitology. 2003;127:571–577. doi: 10.1017/s0031182003004050. [DOI] [PubMed] [Google Scholar]
- Roche D.G., Leung B., Franco E.F.M., Torchin M.E. Higher parasite richness, abundance and impact in native versus introduced cichlid fishes. Int. J. Parasitol. 2010;40:1525–1530. doi: 10.1016/j.ijpara.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Rode N.O., Lievens E.J., Segard A., Flaven E., Jabbour-Zahab R., Lenormand T. Cryptic microsporidian parasites differentially affect invasive and native Artemia spp. Int. J. Parasitol. 2013;43:795–803. doi: 10.1016/j.ijpara.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Roy H.E., Handley L.J.L., Schönrogge K., Poland R.L., Purse B.V. Can the enemy release hypothesis explain the success of invasive alien predators and parasitoids? BioControl. 2011;56:573–595. [Google Scholar]
- Sala O.E., Chapin F.S., Armesto J.J., Berlow E., Bloomfield J., Dirzo R., Huber-Sanwald E., Huenneke L.F., Jackson R.B., Kinzig A., Leemans R., Lodge D.M., Mooney H.A., Oesterheld M., Poff N.L., Sykes M.T., Walker B.H., Walker M., Wall D.H. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Sánchez M.I., Rode N.O., Flaven E., Redón S., Amat F., Vasileva G.P., Lenormand T. Differential susceptibility to parasites of invasive and native species of Artemia living in sympatry: consequences for the invasion of A. franciscana in the Mediterranean region. Biol. Invasions. 2012;14:1819–1829. [Google Scholar]
- Simberloff D., Martin J.L., Genovesi P., Maris V., Wardle D.A., Aronson J., Courchamp F., Galil B., García-Berthou E., Pascal M., Pyšek P., Sousa R., Tabacchi E., Vilà M. Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 2013;28:58–66. doi: 10.1016/j.tree.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Simon K.S., Townsend C.R. Impacts of freshwater invaders at different levels of ecological organisation, with emphasis on salmonids and ecosystem consequences. Freshw. Biol. 2003;48:982–994. [Google Scholar]
- Slothouber Galbreath J.G.M., Smith J.E., Becnel J.J., Butlin R.K., Dunn A.M. Reduction in post-invasion genetic diversity in Crangonyx pseudogracilis (Amphipoda: Crustacea): a genetic bottleneck or the work of hitchhiking vertically transmitted microparasites? Biol. Invasions. 2010;12:191–209. [Google Scholar]
- Slothouber Galbreath J.G.M., Smith J.E., Terry R.S., Becnel J.J., Dunn A.M. Invasion success of Fibrillanosema crangonycis, n. sp., ng: a novel vertically transmitted microsporidian parasite from the invasive amphipod host Crangonyx pseudogracilis. Int. J. Parasitol. 2004;34:235–244. doi: 10.1016/j.ijpara.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder W.E., Evans E.W. Ecological effects of invasive arthropod generalist predators. Annu. Rev. Ecol. Evol. Syst. 2006;37:95–122. [Google Scholar]
- Starkie A. Management issues relating to the European eel Anguilla anguilla. Fish. Manag. Ecol. 2003;10:361–364. [Google Scholar]
- Stentiford G.D., Feist S.W., Stone D.M., Bateman K.S., Dunn A.M. Microsporidia: diverse, dynamic, and emergent pathogens in aquatic systems. Trends Parasitol. 2013;29:567–578. doi: 10.1016/j.pt.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Strauss A., White A., Boots M. Invading with biological weapons: the importance of disease-mediated invasions. Funct. Ecol. 2012;26:1249–1261. [Google Scholar]
- Strayer D.L. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010;55:152–174. [Google Scholar]
- Streicker D.G., Fenton A., Pedersen A.B. Differential sources of host species heterogeneity influence the transmission and control of multihost parasites. Ecol. Lett. 2013;16:975–984. doi: 10.1111/ele.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain L., Perrot-Minnot M.J., Cézilly F. Differential influence of Pomphorhynchus laevis (Acanthocepahala) on brain serotonergic activity in two congeneric host species. Biol. Lett. 2007;3:68–71. doi: 10.1098/rsbl.2006.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschewski H. Hosts and parasites as aliens. J. Helminthol. 2006;80:99–128. doi: 10.1079/joh2006364. [DOI] [PubMed] [Google Scholar]
- Telfer S., Bown K.J. The effects of invasion on parasite dynamics and communities. Funct. Ecol. 2012;26:1288–1299. [Google Scholar]
- Telfer S., Bown K.J., Seules R., Begon M., Hayden T., Birtles R. Disruption of a host-parasite system following the introduction of an exotic host species. Parasitology. 2005;130:661–668. doi: 10.1017/s0031182005007250. [DOI] [PubMed] [Google Scholar]
- Thieltges D.W., Hussel B., Herrmann J., Jensen K.T., Krakau M., Taraschewski H., Reise K. Parasites in the northern Wadden Sea: a conservative ecosystem component over 4 decades. Helgol. Mar. Res. 2008;62:37–47. [Google Scholar]
- Thieltges D.W., Reise K., Prinz K., Jensen K.T. Invaders interfere with native parasite-host interactions. Biol. Invasions. 2009;11:1421–1429. [Google Scholar]
- Tompkins D., Dunn A.M., Smith M.J., Telfer S. Wildlife diseases: from individuals to ecosystems. J. Anim. Ecol. 2011;80:19–38. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- Torchin M.E., Lafferty K.D. Escape from parasites. In: Rilov G., Crooks J., editors. Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives. Springer Verlag; Berlin: 2009. pp. 203–214. [Google Scholar]
- Torchin M.E., Lafferty K.D., Dobson A.P., Mckenzie V.J., Kuris A.M. Introduced species and their missing parasites. Nat. Lond. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Torchin M.E., Lafferty K.D., Kuris A.M. Infestation of an introduced host, the European green crab, Carcinus maenas, by a symbiotic nemertean egg predator, Carcinonemertes epialti. J. Parasitol. 1996;82:449–453. [PubMed] [Google Scholar]
- Torchin M.E., Lafferty K.D., Kuris A.M. Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol. Invasions. 2001;3:333–345. [Google Scholar]
- Torchin M.E., Lafferty K.D., Kuris A.M. Parasites and marine invasions. Parasitology. 2002;124:S137–S151. doi: 10.1017/s0031182002001506. [DOI] [PubMed] [Google Scholar]
- Torchin M.E., Mitchell C.E. Parasites, pathogens, and invasions by plants and animals. Front. Ecol. Environ. 2004;2:183–190. [Google Scholar]
- Van den Brink F.W.B., Van der Velde G., bij de Vaate A. Amphipod invasion on the Rhine. Nature. 1991;352:576. [Google Scholar]
- Van Riel M.C., Van der Velde G., de Vaate A.B. Pomphorhynchus spec. (Acanthocephala) uses the invasive amphipod Chelicorophium curvispinum (G.O. Sars, 1895) as an intermediate host in the river Rhine. Crustaceana. 2003;76:241–246. [Google Scholar]
- Vasileva G.P., Redón S., Amat F., Nikolov P.N., Sánchez M.I., Lenormand T., Georgiev B.B. Records of cysticercoids of Fimbriarioides tadornae Maksimova, 1976 and Branchiopodataenia gvozdevi (Maksimova, 1988) (Cyclophyllidea: Hymenolepididae) from brine shrimps at the Mediterranean coasts of Spain and France, with a key to cestodes from Artemia spp. from the Western Mediterranean. Acta Parasitol. 2009;54:143–150. [Google Scholar]
- Wattier R.A., Haine E.R., Beguet J., Martin G., Bollache L., Muskó I.B., Platvoet D., Rigaud T. No genetic bottleneck or associated microparasite loss in invasive populations of a freshwater amphipod. Oikos. 2007;116:1941–1953. [Google Scholar]
- Wedekind C., Christen M., Schärer L., Treichel N. Relative helminth size in crustacean hosts: in vivo determination, and effects of host gender and within-host competition in a copepod infected by a cestode. Aquat. Ecol. 2000;34:279–285. [Google Scholar]
- Weekes P.J., Penlington B. First records of Ligula intestinalis (Cestoda) in rainbow trout, Salmo gairdneri, and common bully, Gobiomorphus cotidianus, in New Zealand. J. Fish Biol. 1986;28:183–190. [Google Scholar]
- White E.M., Wilson J.C., Clarke A.R. Biotic indirect effects: a neglected concept in invasion biology. Divers. Distrib. 2006;12:443–455. [Google Scholar]
- Woolhouse M.E., Haydon D.T., Antia R. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. 2005;20:238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetlmeisl C., Hermann J., Petney T., Glenner H., Griffiths C., Taraschewski H. Parasites of the shore crab Carcinus maenas (L.): implications for reproductive potential and invasion success. Parasitology. 2011;138:394–401. doi: 10.1017/S0031182010001344. [DOI] [PubMed] [Google Scholar]