Fig. 2.
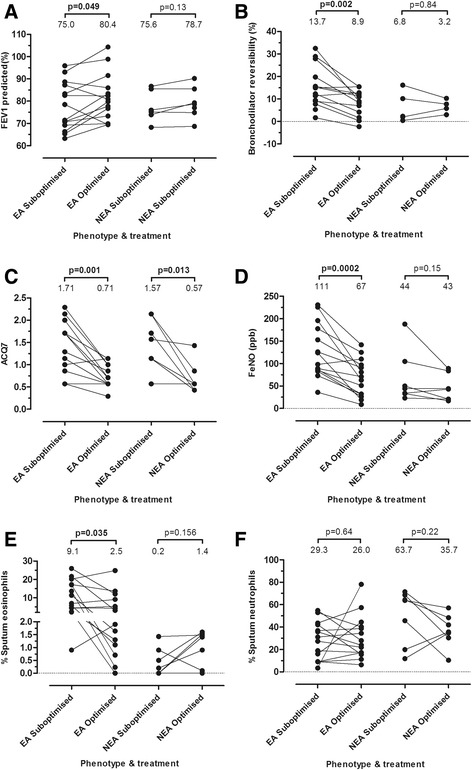
Clinical and sputum sample inflammatory parameters for all CIT participants (n = 21). Data represent the assessment in which treatment was considered as sub-optimised or optimised. a – FEV1% predicted, b- change in FEV1% predicted post-bronchodilator (BDR), c – ACQ7, d- exhaled NO levels, e – change in % sputum eosinophils, f-change in % sputum neutrophils. Median data values are expressed at the top of each group
