Key Clinical Message
We have presented a case of advanced HF, in which newly developed AF hastened the timing of the implantation of mechanical support. Newly developed AF in advanced HF may be intractable by medical therapies and could be a key event that determines the timing of mechanical support.
Keywords: Advanced heart failure, atrial fibrillation, dilated cardiomyopathy, INTERMACS, ventricular assist device
Introduction
Atrial fibrillation (AF) is a very common arrhythmia that can affect clinical outcomes. In cases of heart failure (HF), the prevalence of AF as a comorbidity has been reported 1. Previous studies have suggested that the presence of AF leads to an impaired left ventricular (LV) systolic function 2, 3; however, the precise effects of AF on patients with HF remain to be elucidated. In this case report, we studied the effects of AF in a patient with dilated cardiomyopathy (DCM), who required the implantation of a LV assist device (LVAD) for advanced refractory HF. The presentation conformed to the Declaration of Helsinki and was reviewed and approved by the University of Tokyo Institutional Review Board (2650). Written informed consent was obtained from the subject.
Case
Because of a family history of DCM, the 38‐year‐old male patient underwent echocardiography 12 years ago and was subsequently diagnosed with HF because of DCM. At that time, he began a regimen of beta blockers, which stabilized his condition for 10 years. However, 2 years ago, his HF deteriorated to New York Heart Association functional class III, and he underwent cardiac resynchronization therapy with defibrillation. His medications included carvedilol 20 mg, imidapril 2.5 mg, spironolactone 50 mg, and furosemide 80 mg. In spite of these interventions, his condition gradually worsened. After beginning tolvaptan therapy, he was rehospitalized because of the exacerbation of his HF. The use of catecholamines relieved his symptoms temporarily, but the required dose increased because of organ dysfunction from low perfusion. He was eventually transferred to our hospital to be considered for heart transplantation.
At admission, his blood pressure was low (88/60 mmHg), even after catecholamine administration (2 μg/kg/min dopamine and 4 μg/kg/min dobutamine). His electrocardiogram showed evidence of atrial sensing and ventricular pacing, and his heart rate was 108 bpm. A chest X‐ray showed signs of cardiomegaly and slight congestion. A laboratory examination revealed moderate increases in liver enzyme levels (aspartate transaminase [AST] 146 IU/L, alanine aminotransferase [ALT] 609 IU/L, and total bilirubin [TB] 1.2 mg/dL; Fig. 1).
Figure 1.
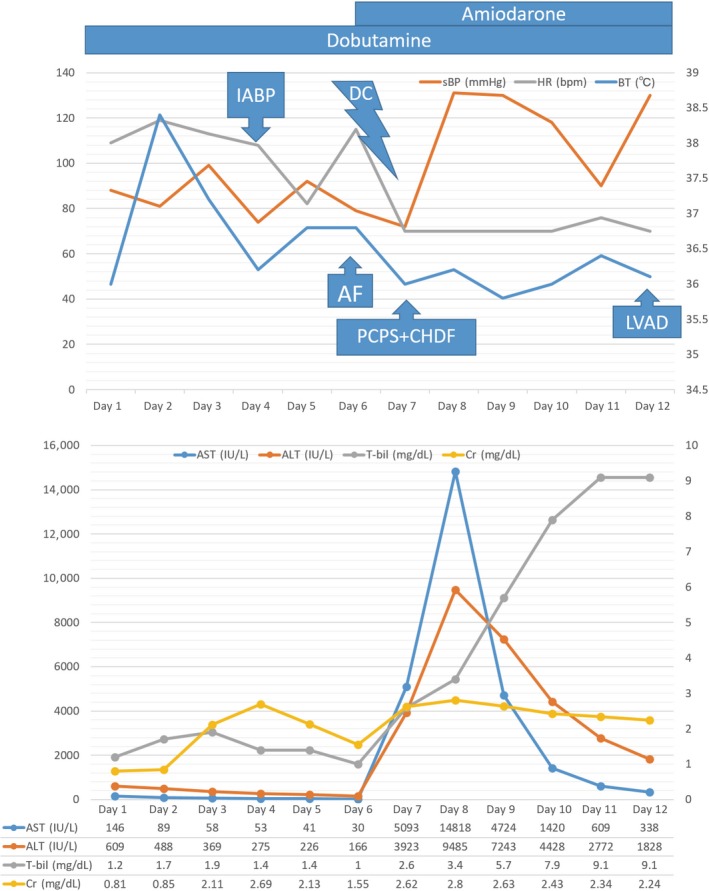
These graphs show the vital parameters along with the liver and renal functions over time. On day 3 after hospitalization, a catheter infection caused sinus tachycardia and increased creatinine and total bilirubin levels. On day 4, an intra‐aortic balloon pump was inserted. As a result, the heart rate decreased, and the liver and renal dysfunctions improved, suggesting an improvement in HF. On day 6, AF occurred, resulting in an abrupt reduction in blood pressure and the progression of liver and renal dysfunctions again in addition to the increase of blood lactate level. The administration of amiodarone did not lead to the recovery of sinus rhythm, and electrocardioversion was performed and stopped the AF. However, the liver enzymes increased significantly, leading to the requirement for mechanical support. On day 12, an extracorporeal ventricular assist device was implanted.
The following day, a fever, possibly from a catheter infection, caused his HF to deteriorate, leading to oliguria and progressive worsening of liver function (TB: 1.2→1.9 mg/dL, creatinine [Cre]: 0.81→2.11 mg/dL). We determined that the condition was intractable by the increase in catecholamine doses and inserted an intra‐aortic balloon pump (IABP). Before the insertion of IABP, central venous oxygen saturation level had reduced to 37%, which suggested severe hemodynamic collapse. However, the IABP worked effectively, suppressing the progression of organ dysfunction (TB: 1.9→1.0 mg/dL, Cre: 2.11→1.55 mg/dL). However, AF occurred soon afterward, and severe liver and renal dysfunctions progressed rapidly (AST: 41→686 IU/L, ALT: 226→774 IU/L, TB: 1.0→1.5 mg/dL, Cre: 1.55→1.86 mg/dL). Firstly, we tried administering amiodarone, but this did not lead to the recovery of sinus rhythm. The lactate concentration gradually increased (0.7→1.5→2.2 mmol/L); therefore, rapid hemodynamic stabilization was considered to be warranted as soon as possible. Electrocardioversion was performed, and sinus rhythm was achieved. However, even after the AF ceased, the progression of organ dysfunction could not be suppressed, and severe liver and kidney dysfunctions were observed (AST: 686→14,818 IU/L, ALT: 774→9485 IU/L, TB: 1.5→3.4 mg/dL, Cre: 1.86→3.23 mg/dL). For these hemodynamic derangements, we finally began percutaneous cardiopulmonary support (PCPS) and continuous hemodiafiltration. After 5 days, an extracorporeal ventricular assist device (VAD) (Nipro VAD; Nipro Corporation, Osaka, Japan) was implanted, after which the patient's liver dysfunction gradually improved. We subsequently replaced the extracorporeal VAD with an implantable VAD after the patient was listed for heart transplantation.
Discussion
This case represents the effects of AF on the course of advanced HF in a patient with DCM, who was a candidate for LVAD or heart transplantation 4, 5. The occurrence of AF in a patient with HF can exhibit various effects in different situations. However, there are only limited reports addressing the effects of newly developed AF on patients with medically intractable, advanced HF, who are candidates for LVAD therapy. The effect of AF in patients with HF increases with the increase in the severity of HF 6, particularly with respect to the impairment in LV systolic function 3. Some reports have stated that there is no impact of AF on the course of HF 7. In contrast, a newly developed AF, as presented in the current case, more significantly impacts the course of HF than an existing AF 8, 9, 10. According to the mechanism of it, the onset of AF certainly has a negative impact on heart function because of an increased mitral and tricuspid regurgitation and the negative impact on LV function 11, 12, 13, 14, 15, 16.
For the factors negatively affecting HF, different therapeutic strategies and treatments should be considered. The balance of the beneficial effects and risk of therapy against AF should be coordinated. In the presence of LV dysfunction, impairments in hemodynamic stability caused by AF are significant; therefore, the recovery of a normal sinus rhythm should be pursued more vigorously. Indeed, the beneficial effect of the recovery of sinus rhythm may be enhanced as the severity of HF increases; however, the risk of the associated therapies is also increased. Therefore, the issue of rhythm or rate control becomes a more difficult problem as the severity of HF increases 10, 17.
The heart rhythm can be controlled by both pharmacological therapies and electrical interventions. With respect to the antiarrhythmic drugs for the maintenance of sinus rhythm in patients with HF or reduced ejection fraction, the available agents are limited and the most commonly used pharmacological agent is amiodarone 18, 19, 20. However, its depressive effects on myocardial function are emphasized in patients with an impaired systolic function. Torp–Pedersen et al. have demonstrated that treatment with amiodarone is associated with an increased risk of death from HF 11. Another therapeutic agent, dronedarone, may be useful for some patients but should not be used for patients with severe HF 21. On the other hand, myocardial injury following electrocardioversion may have a negative impact on the course of HF 22. Indeed, electrical shock leads to an increased troponin level and an irreversible decline in cardiac function, resulting in an increased risk of all‐cause mortality 23, 24. The fact that the most common cause of death among patients who received an ICD shock was progressive HF supports this hypothesis 25. In these ways, the rhythm control strategies (both pharmacological and electrical) can have a negative impact on the course of HF, which may be enhanced in the cases of patients with severely impaired systolic function.
On the other hand, an urgent coordination of heart rate may delay the progression of HF. But, the heart‐rate‐lowering agents also require close monitoring because of their negative impact on LV function. Digoxin has been used previously for the control of heart rate in patients with a reduced ejection fraction because of its beneficial effects on ventricular function 26. However, several studies have reported conflicting evidences about the efficacy of digoxin in such patients 27. One study proposed the use of landiolol to safely reduce the heart rate without suppressing cardiac function in patients with a reduced ejection fraction as well as in patients with severe LV dysfunction 28. Another study suggested that landiolol was more effective for urgent heart rate control than digoxin and did not increase the incidence of adverse events 29. These rate control therapeutics may have comparatively smaller effects than rhythm control therapies.
On the other hand, AF can be a marker of advanced diseases during the clinical course of DCM 30, and it has become the most critical factor in determining the appropriate timing of mechanical supports such as ECMO. INTERMACS patient profiles were devised to categorize patients for the purpose of risk assessment before LVAD implantation 5. The classification of INTERMACS profile is important for patients with advanced HF because the profile can predict the outcome after LVAD implantation and serve as a useful reference for the appropriate timing of mechanical supports, including LVAD. INTERMACS profile 1 or 2 has been reported to be the worst prognosis following LVAD implantation 31, and the implantation of LVAD should be performed before the patient's INTERMACS profile progresses to profile 1 or 2. The INTERMACS profile has some modifying factors to improve patient characterization such as temporary circulatory support. However, there are no reports on perioperative AF in patients with advanced HF and eligible for the implantation of LVAD. In such a situation, the most appropriate therapy against AF appears to be the rapid preparation for the implantation of LVAD as soon as possible. Therefore, newly developed AF may have modifying effects on the INTERMACS profiles of some patients with advanced HF. However, we have yet to elucidate which patients are vulnerable to the burden of newly developed AF like this patient.
We reviewed recent cases of nonischemic cardiomyopathy, in which newly developed onset of AF hastened the timing of the implantation of mechanical support, leading to the implantation of LVAD finally (Table 1) 32. Various treatment strategies against AF were used, including amiodarone and electrical cardioversion, but no other interventions were effective, than the early induction of IABP or PCPS, as described in the current case. Interestingly, the sinus rhythm was recovered in all cases after LVAD implantation, suggesting that surgical interventions were most effective therapies in these situations. Therefore, the newly developed AF means urgent requirement of next surgical circulatory support including LVAD. It may suggest that in some cases, a rhythm therapy cannot stop the progression of HF even if the recovery of sinus rhythm is achieved or the burden of rhythm therapy deteriorates the course of HF. All of the cases examined had moderate or severe mitral regurgitation except for case 1, who was in the dilated phase of hypertrophic cardiomyopathy. These characteristics suggest that an association between the presence of mitral regurgitation and the vulnerability to the burden of newly developed AF. Similar to mitral regurgitation, left atrial volume is also thought to be a prognostic indicator in patients with DCM 33 and the prognostic value of left atrial volume supports the validity of AF as a prognostic marker for the deterioration of HF. On the other hand, AF can be classified by a working histological/pathophysiological classification scheme 34. According to this criteria, the restorations of sinus rhythm after LVAD implantation in these cases suggested AF was associated with HF rather than myocardial injuries derived from cardiomyopathy. The accurate identification of the cause of AF may help the prediction of burden of it. However, the characteristics that can accurately predict the deterioration of advanced HF by newly developed AF need to be investigated more vigorously.
Table 1.
Recent cases of nonischemic cardiomyopathy in which newly developed onset of AF hastened the timing of the implantation of mechanical support
| Age | Sex | Disease | Valve | Time 1 | Mechanical support | Time 2 | LVAD | Treatment | |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 28 | Male | D‐HCM | None | 8 d | IABP | 10 d | Jarvik 2000 | Electrical defibrillation, Landiolol |
| Case 2 | 21 | Male | DCM | Severe MR | 9 d | IABP | 13 d | Heart MateI II | Electrical defibrillation, Amiodarone, Landiolol, Digitalis |
| Case 3 | 46 | Male | DCM | Moderate MR | 6 d | PCPS | 7 d | NIPRO VAD | Electrical defibrillation, Amiodarone |
| Case 4 | 53 | Male | DCM | Severe MR | 1 m | IABP | 2 m | Jarvik 2000 | Amiodarone, Digitalis |
| Case 5 | 42 | Male | DCM | Severe MR | 9 d | IABP | 16 d | Heart Mate II | Amiodarone, Landiolol |
| Present case | 38 | Male | DCM | Severe MR | 1 d | IABP, PCPS | 9 d | NIPRO VAD | Amiodarone, Landiolol, Electrical difibrillation |
All cases recovered to sinus rhythm or pacemaker rhythm after LVAD implantation. (i) Time 1 is the interval from occurrence of AF to mechanical support (e.g., IABP, PCPS). (ii) Time 2 is the interval from occurrence of AF to LVAD implantation.
D‐HCM, dilated phase of hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; MR, mitral regurgitation; d, days; m, months; IABP, intra‐aortic balloon pumping; PCPS, percutaneous cardiopulmonary support; LVAD, left ventricular assist device.
Conclusion
We have presented a case of advanced HF, in which newly developed AF hastened the timing of the implantation of mechanical support. Newly developed AF could be a key event that induces a deterioration in the INTERMACS profile.
Authorship
AS and EA: wrote the manuscript. MH, YH, HM, DN, SM, MW, and IK: reviewed the manuscript.
Conflict of Interest
None declared.
Clinical Case Reports 2017; 5(12): 2028–2033
References
- 1. Lubitz, S. A. , Benjamin E. J., and Ellinor P. T.. 2010. Atrial fibrillation in congestive heart failure. Heart Fail. Clin. 6:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mamas, M. A. , Caldwell J. C., Chacko S., Garratt C. J., Fath‐Ordoubadi F., Neyses L.. 2009. A meta‐analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur. J. Heart Fail. 11:676–683. [DOI] [PubMed] [Google Scholar]
- 3. Cheng, M. , Lu X., Huang J., Zhang J., Zhang S., Gu D.. 2014. The prognostic significance of atrial fibrillation in heart failure with a preserved and reduced left ventricular function: insights from a meta‐analysis. Eur. J. Heart Fail. 16:1317–1322. [DOI] [PubMed] [Google Scholar]
- 4. Miller, L. W. , and Guglin M.. 2013. Patient selection for ventricular assist devices: a moving target. J. Am. Coll. Cardiol. 61:1209–1221. [DOI] [PubMed] [Google Scholar]
- 5. Stevenson, L. W. , Pagani F. D., Young J. B., Jessup M., Miller L., Kormos R. L., et al. 2009. INTERMACS profiles of advanced heart failure: the current picture. J. Heart Lung Transplant. 28:535–541. [DOI] [PubMed] [Google Scholar]
- 6. Maisel, W. H. , and Stevenson L. W.. 2003. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 91:2D–8D. [DOI] [PubMed] [Google Scholar]
- 7. Mahoney, P. , Kimmel S., DeNofrio D., Wahl P., and Loh E.. 1999. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. Am. J. Cardiol. 83:1544–1547. [DOI] [PubMed] [Google Scholar]
- 8. Pozzoli, M. , Cioffi G., Traversi E., Pinna G. D., Cobelli F., Tavazzi L.. 1998. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J. Am. Coll. Cardiol. 32:197–204. [DOI] [PubMed] [Google Scholar]
- 9. Swedberg, K. , Olsson L. G., Charlesworth A., Cleland J., Hanrath P., Komajda M., et al. 2005. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long‐term treatment with beta‐blockers: results from COMET. Eur. Heart J. 26:1303–1308. [DOI] [PubMed] [Google Scholar]
- 10. Anter, E. , Jessup M., and Callans D. J.. 2009. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation 119:2516–2525. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed, A. , and Perry G. J.. 2005. Incident atrial fibrillation and mortality in older adults with heart failure. Eur. J. Heart Fail. 7:1118–1121. [DOI] [PubMed] [Google Scholar]
- 12. Zipes, D. P. 1997. Atrial fibrillation. A tachycardia‐induced atrial cardiomyopathy. Circulation 95:562–564. [DOI] [PubMed] [Google Scholar]
- 13. Han, F. T. , Kiser R., Nixon J. V., Wood M. A., and Ellenbogen K. A.. 2011. What is the time course of reversal of tachycardia‐induced cardiomyopathy? Europace 13:139–141. [DOI] [PubMed] [Google Scholar]
- 14. van den Berg, M. P. , van Gelder I. C., and van Veldhuisen D. J.. 2002. Impact of atrial fibrillation on mortality in patients with chronic heart failure. Eur. J. Heart Fail. 4:571–575. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong, P. W. , Stopps T. P., Ford S. E., and de Bold A. J.. 1986. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation 74:1075–1084. [DOI] [PubMed] [Google Scholar]
- 16. Jeong, Y. H. , Choi K. J., Song J. M., Hwang E. S., Park K. M., Nam G.‐B., et al. 2008. Diagnostic approach and treatment strategy in tachycardia‐induced cardiomyopathy. Clin. Cardiol. 31:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talajic, M. , Khairy P., Levesque S., Connolly S. J., Dorian P., Dubuc M., et al. 2010. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J. Am. Coll. Cardiol. 55:1796–1802. [DOI] [PubMed] [Google Scholar]
- 18. Roy, D. , Talajic M., Nattel S., Wyse D. G., Dorian P., Lee K. L., et al. 2008. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 19. Singh, B. N. , Singh S. N., Reda D. J., Tang X. C., Lopez B., Harris C. L., et al. 2005. Amiodarone versus sotalol for atrial fibrillation. N. Engl. J. Med. 352:1861–1872. [DOI] [PubMed] [Google Scholar]
- 20. Deedwania, P. C. , Singh B. N., Ellenbogen K., Fisher S., Fletcher R., Singh S. N.. 1998. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF‐STAT). The Department of Veterans Affairs CHF‐STAT Investigators. Circulation 98:2574–2579. [DOI] [PubMed] [Google Scholar]
- 21. Kober, L. , Torp‐Pedersen C., McMurray J. J., Gotzsche O., Levy S., Crijns H., et al. 2008. Increased mortality after dronedarone therapy for severe heart failure. N. Engl. J. Med. 358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 22. Sweeney, M. O. , Sherfesee L., DeGroot P. J., Wathen M. S., and Wilkoff B. L.. 2010. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter‐defibrillator patients. Heart Rhythm 7:353–360. [DOI] [PubMed] [Google Scholar]
- 23. Daubert, J. P. , Zareba W., Cannom D. S., McNitt S., Rosero S. Z., Wang P., et al. 2008. Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J. Am. Coll. Cardiol. 51:1357–1365. [DOI] [PubMed] [Google Scholar]
- 24. Sham'a, R. A. , Nery P., Sadek M., Yung D., Redpath C., Perrin M., et al. 2014. Myocardial injury secondary to ICD shocks: insights from patients with lead fracture. Pacing Clin. Electrophysiol. 37:237–241. [DOI] [PubMed] [Google Scholar]
- 25. Poole, J. E. , Johnson G. W., Hellkamp A. S., Anderson J., Callans D. J., Raitt M. H., et al. 2008. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med. 359:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khand, A. U. , Rankin A. C., Martin W., Taylor J., Gemmell I., Cleland J. G. F.. 2003. Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J. Am. Coll. Cardiol. 42:1944–1951. [DOI] [PubMed] [Google Scholar]
- 27. Whitbeck, M. G. , Charnigo R. J., Khairy P., Ziada K., Bailey A. L., Zegarra M. M., et al. 2013. Increased mortality among patients taking digoxin – analysis from the AFFIRM study. Eur. Heart J. 34:1481–1488. [DOI] [PubMed] [Google Scholar]
- 28. Adachi, T. , Sato A., Baba M., Hiraya D., Hasegawa T., Kuroki K., et al. 2014. Novel use of the ultra‐short‐acting intravenous beta 1‐selective blocker landiolol for supraventricular tachyarrhythmias in patients with congestive heart failure. Heart Vessels 29:464–469. [DOI] [PubMed] [Google Scholar]
- 29. Nagai, R. , Kinugawa K., Inoue H., Atarashi H., Seino Y., Yamashita T., et al. 2013. Urgent management of rapid heart rate in patients with atrial fibrillation/flutter and left ventricular dysfunction: comparison of the ultra‐short‐acting beta1‐selective blocker landiolol with digoxin (J‐Land Study). Circ. J. 77:908–916. [DOI] [PubMed] [Google Scholar]
- 30. Zhao, L. , Xu K., Jiang W., Zhou L., Wang Y., Zhang X., et al. 2015. Long‐term outcomes of catheter ablation of atrial fibrillation in dilated cardiomyopathy. Int. J. Cardiol. 190:227–232. [DOI] [PubMed] [Google Scholar]
- 31. Kirklin, J. K. , Naftel D. C., Pagani F. D., Kormos R. L., Stevenson L. W., Blume E. D., et al. 2014. Sixth INTERMACS annual report: a 10,000‐patient database. J. Heart Lung Transplant. 33:555–564. [DOI] [PubMed] [Google Scholar]
- 32. Cowger, J. , Shah P., Stulak J., Maltais S., Aaronson K. D., Kirklin J. K., et al. 2016. INTERMACS profiles and modifiers: heterogeneity of patient classification and the impact of modifiers on predicting patient outcome. J. Heart Lung Transplant. 35:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossi, A. , Cicoira M., Zanolla L., Sandrini R., Golia G., Zardini P., et al. 2002. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 40:1425. [DOI] [PubMed] [Google Scholar]
- 34. Goette, A. , Kalman J. M., Aguinaga L., Akar J., Cabrera J. A., Chen S. A., et al. 2016. EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial cardiomyopathies: definition, characterisation, and clinical implication. J. Arrhythm. 32:247–278. [DOI] [PMC free article] [PubMed] [Google Scholar]


