Abstract
Aims
To investigate if urinary AQP5 serves as a new potential biomarker of diabetic nephropathy.
Methods
Using an AQP5-specific enzyme-linked immunosorbent assay, we measured serum and urine AQP5 first in a cohort consisting of normal controls (n = 26) and patients with diabetes mellitus (n = 25) or diabetic nephropathy (n = 33) and then in a validation cohort possessing normal controls (n = 10), patients with diabetes mellitus (n = 10) or diabetic nephropathy (n = 14), and patients with chronic kidney disease of unknown etiology (n = 10). We used various statistical methods including Pearson’s correlation coefficient, ANOVA with Holm–Sidak test, Receiver Operator Curve, and multiple logistic regression to analyze the data.
Results
Urine AQP5/creatinine 1) is significantly higher in diabetic nephropathy than in other two groups, and in diabetic nephropathy stage V than in stage III; 2) correlates with serum creatinine, urine albumin, and multiple other known risk factors of the disease; and 3) improves the clinical models in distinguishing diabetic nephropathy from normal controls and diabetic mellitus.
Conclusion
Our data suggest that urine AQP5/creatinine may possess diagnostic and prognostic values as a biomarker of diabetic nephropathy.
Keywords: Water channels, Diabetes, Gene expression, Kidney biopsy, Urinary biomarker
1. Introduction
Diabetic nephropathy (DN) is clinically characterized by persistent albuminuria in the setting of diabetes. Elevated arterial blood pressure and progressively decreased glomerular filtration rate (GFR) are common features associated with the disease. Patients with DN may also have a high risk of cardiovascular disease and develop retinopathy. The prevalence of DN among diabetic patients has not decreased in the last 2 decades (Gohda & Tomino, 2013). The incidence of DN in type I diabetes is more than 20%–30% (Ruggenenti, Schieppati, & Remuzzi, 2001). DN is the most common single cause of end-stage renal disease and one of the most significant long-term complications associated with diabetes in the U.S (Molitch et al., 2004).
Currently, the only routine laboratorial markers of DN are estimated GFR and proteinuria/albuminuria. Albuminuria has been considered a sine qua non condition for the diagnosis of DN and has been widely used as a surrogate outcome of chronic kidney disease (CKD). However, some patients of type 1 diabetes can develop kidney disease even though their urinary albumin levels are in the normal range (Perkins, Ficociello, Roshan, Warram, & Krolewski, 2010), suggesting the limitation of albuminuria alone as a diagnostic and prognostic marker of DN.
Several tubular markers are associated with microalbuminuria in DN and in patients with normoalbuminuria (Galanti, Jamart, Dell’omo, & Donckier, 1996; Pontuch, Toserova, Vozar, Bulas, & Kratochvilova, 1995; Salem, el-Habashy, Saeid, el-Tawil, & Tawfik, 2002; Watts, Powell, Rowe, & Shaw, 1989), implying that tubular malfunction could be an earlier indicator of DN than microalbuminuria. Moreover, tubular markers are also correlated to glycemic control (Pontuch, Jensen, Deckert, Ondrejka, & Mikulecky, 1992; Salem et al., 2002) and diabetic retinopathy (Holm, Nielsen, & Hemmingsen, 1994). Recently, the urine and plasma proteome analyses have led to identification of new markers including haptoglobin, kininogen, and Fetuin-A (Bhensdadia et al., 2013; Inoue et al., 2013; Merchant et al., 2013). However, none of these markers has been incorporated into the clinical setting due to various limitations.
Water channel AQP5 plays a role in the generation of saliva, tears, and pulmonary secretions (Da Silva et al., 2006). It is not detectable by immunoblotting in normal mouse and human kidneys (Krane, Towne, & Menon, 1999; Wu et al., 2013), indicating that AQP5 plays little role in normal renal physiology. We have reported that AQP5 is upregulated in kidney biopsies from patients with DN, where it interacts with AQP2 at the peri-nuclear region. The interaction was validated in multiple assays (Wu et al., 2013). Overexpression of AQP5 in IMCD3 cells led to impaired AQP2 membrane localization as evidenced by cell surface biotin assays (Wu et al., 2013). However, if AQP5 is detectable in urine and if urine AQP5 can serve as a novel potential marker of DN remain completely unknown. In this study, we performed AQP5-specific enzyme-linked immunosorbent assay (ELISA) to address these questions. Our data suggest that urine AQP5 is a novel marker of DN.
2. Materials and methods
2.1. Reagents
Human AQP5 ELISA kits (Uscn Life Science) were used according to the manufacturer’s instruction.
2.2. Study population
For the diabetic mellitus (DM) and DN groups, the inclusion criteria are 1) ≥25 years old; and 2) diagnosed with type 2 DM with various stages of normo-, micro-, and macro-albuminuria; The exclusion criteria are 1) pregnancy; 2) presence of primary glomer-ulonephritis or other kidney-damaging disease such as autoimmune disease or hepatitis B; 3) conditions of urinary tract infection or severe heart failure which affect urinary albumin excretion; and 4) presence of other primary medical conditions including cancer. Normal controls are defined as those who have no evidence of any medical conditions and display normal albumin/creatinine ratio (ACR) and no microalbuminuria. Diagnosis was based on the diabetic history, laboratory evaluation, imaging of the kidneys, and physical examination, especially if retinopathy and peripheral neuropathy were present. DM was diagnosed according to the criterion established by the WHO diabetic committee of experts in 1999. DN was defined as the presence of persistent ‘clinical’ albuminuria (albumin excretion rate (AER) >300 mg/24 h) in a patient with diabetes for over 5 years, in the absence of urinary tract infection (UTI), other renal diseases, or heart failure (Thomas, 2010). DN Stages III and IV were assigned according to presence of microalbuminuria (ACR: 30 to 300 μg/mg) and macroalbuminuria (ACR: >300 μg/mg), respectively. Stage V was diagnosed by increased serum creatinine (≥175 μmol/L), increased blood pressure, development of end-stage renal failure, or requirement of dialysis or transplantation (Adler et al., 2003; Mollsten et al., 2011; Yokoyama, Myrup, Rossing, & Ostergaard, 1996). Efforts were made to collect various clinical parameters (Table 1). Among them are age, albumin, total protein, GFR, body mass index, systolic blood pressure, diastolic blood pressure, ACR, glycated haemoglobin, low density lipoprotein, hemoglobin, hematocrit, blood urea nitrogen, serum creatinine, uric acid, alanine aminotransferase, aspartate transaminase, total bilirubin, direct bilirubin, total biliary acid, total glycerides, and fasting blood glucose. It should be noted that while some of these parameters were collected in all subjects examined, others were available only in a subset of patients.
Table 1.
Clinical and laboratorial variables for all subjects.
| All subjects | Normal | DM | DN | |
|---|---|---|---|---|
| Number of subjects | 84 | 26 | 25 | 33 |
| Age, yrs | 53 ± 12.6 | 44.8 ± 11.8 | 54.92 ± 8.6* | 58.9 ± 12.2* |
| Gender, male/female | 41/39 | 11/15 | 14/11 | 16/13 |
| Duration of diabetes, yrs | 8.38 ± 4.8 | NA | 6.6 ± 4.4 | 9.9 ± 4.7** |
| SBP, mmHg, | 138.3 ± 21.2 | NA | 132.8 ± 19.0 | 143.0 ± 22.2 |
| DBP, mmHg | 82.2 ± 11.2 | NA | 82.8 ± 11.1 | 81.7 ± 11.4 |
| Body mass index, kg/m2 | 24.14 ± 4 | NA | 24.8 ± 3.7 | 23.52 ± 4 |
| Estimated GFR | 100.8 ± 39.0 | 112.9 ± 22.2 | 115.5 ± 24.2 | 78.8 ± 49.0*,** |
| Serum albumin, g/l | 39.6 ± 7.1 | 45.1 ± 3.7 | 39.7 ± 5.1* | 34.3 ± 7.2*,** |
| Blood glucose, mmol/l | 7.6 ± 3.3 | 4.9 ± 0.4 | 8.2 ± 2.2* | 9.5 ± 3.9* |
| HbA1c, % | 8.9 ± 2.4 | NA | 9.0 ± 2.3 | 8.8 ± 2.6 |
| Total cholesterol, mmol/l | 4.8 ± 1.1 | 5.1 ± 0.9 | 4.6 ± 0.1 | 4.8 ± 1.3 |
| LDL cholesterol, mmol/l | 2.9 ± 1.1 | 3.3 ± 0.6 | 2.7 ± 1.0 | 2.8 ± 1.3 |
| Triglycerides, mmol/l | 1.7 ± 1.4 | 1.0 ± 0.5 | 2.1 ± 1.6* | 2.0 ± 1.6* |
| HGB (g/L) | 124.0 ± 19.1 | 129.4 ± 11.7 | 129.0 ± 17.1 | 115.3 ± 22.4*,** |
| PLT (×109/L) | 187.1 ± 53.6 | 193.1 ± 41.6 | 189.9 ± 62.8 | 180.2 ± 54.6 |
| HCT (%) | 35.4 ± 7.8 | 36.6 ± 3.0 | 36.7 ± 9.1 | 33.5 ± 8.7 |
| BUN (mmol/L) | 5.6 ± 3.3 | 4.0 ± 1.1 | 5.07 ± 1.8* | 7.3 ± 4.6*,** |
| SCr (μmol/L) | 108 ± 103 | 69 ± 14 | 73 ± 16 | 168 ± 148*,** |
| UA (mmol/L) | 321.8 ± 92.1 | 320.7 ± 86.0 | 314.4 ± 110.3 | 329.1 ± 81.4 |
| Globulin (g/L) | 26.8 ± 4.1 | 26.6 ± 2.9 | 25.5 ± 4.5 | 28.2 ± 4.3** |
| β2-microglobulin (mg/L) | 3.6 ± 4.6 | NA | 1.4 ± 0.5 | 6.5 ± 6.0** |
| Cystatin C (mg/L) | 1.6 ± 1.6 | NA | 0.9 ± 0.3 | 2.5 ± 2.2** |
| K (mmol/L) | 3.8 ± 0.5 | NA | 3.7 ± 0.4 | 3.9 ± 0.5 |
| Na (mmol/L) | 137.1 ± 17.4 | NA | 139.6 ± 3.6 | 135.0 ± 23.5 |
| Ca (mmol/L) | 2.2 ± 0.1 | NA | 2.2 ± 0.1 | 2.2 ± 0.2 |
| P (mmol/L) | 1.2 ± 0.2 | NA | 1.1 ± 0.3 | 1.2 ± 0.2 |
| ALT (U/L) | 20.7 ± 10.9 | 19.8 ± 9.1 | 23.5 ± 14.8 | 19.1 ± 7.7 |
| AST (U/L) | 22.0 ± 7.2 | NA | 21.8 ± 7.2 | 22.3 ± 7.3 |
| TBIL (μmol/L) | 10.8 ± 5.7 | 12.2 ± 7.1 | 12.1 ± 4.7 | 8.3 ± 4.0*,** |
| DBIL (μmol/L) | 3.7 ± 1.7 | 3.7 ± 1.4 | 4.5 ± 1.9 | 3.0 ± 1.4** |
| TBA (μmol/L) | 5.7 ± 6.2 | NA | 6.3 ± 7.6 | 5.2 ± 4.7 |
| Urinary proteins, mg/day | 638.2 ± 1380.0 | NA | 20.3 ± 31.4 | 1128.3 ± 1704.6** |
| Specific gravity of urine | 1.02 ± 0.07 | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.01 |
| Urine PH | 5.9 ± 0.8 | 5.9 ± 0.6 | 5.7 ± 0.8 | 6.0 ± 0.9 |
| 24 h urine volume (ml) | 1496 ± 681 | NA | 1424 ± 534 | 1558 ± 791 |
| ACR (μg/mg) | 1226 ± 403 | NA | 12 ± 7 | 2274 ± 3769** |
Data are means ± SE. GFR: glomerular filtration rate (ml/min/1.73 m2); BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure. ACR: urine albumin-to-creatinine ratio; HbA1c: glycated haemoglobin; LDL: low density lipoprotein; HGB: hemoglobin; PLT: pallet; HCT: hematocrit; BUN: blood urea nitrogen; Scr: serum creatinine; UA: uric acid; ALT: alanine aminotransferase; AST: aspartate transaminase; TBIL: total bilirubin; DBIL: direct bilirubin; TBA: total biliary acid; NA: not available.
p β 0.05 vs. Normal control.
p β 0.05 vs. DM.
All patients were recruited and examined in the XiangYa Hospital, Central South University. This study was conducted with adherence to the Declaration of Helsinki and approved by the local Ethics Committee. All patients signed an informed consent.
2.3. Measurement of AQP5
Urine (10 ml) and blood (2 ml) were collected in the morning. All samples were centrifuged within 2 h after collection for 20′ at 1000 g. Urine supernatant and serum were either used for measurement of clinical parameters and AQP5 or stored as aliquots at −80 °C. AQP5 was determined with the AQP5 ELISA kit, following the manufacturer’s instruction. The manufacturer has verified the high specificity of the AQP5 ELISA kit, with <1% cross reactivity towards AQP1, AQP2, AQP3, AQP4, AQP8, IL-6, TNF-α, TGF-β1, or IFN-γ. We performed spike-and-recovery experiments done in diabetic urine and serum. No significant interference with the assay relating to albumin was found. Our intra-assay or inter-assay variability is <20%.
2.4. Statistical analyses
Correlations between urine AQP5 and clinical parameters were calculated as the Pearson’s correlation coefficient, using Microsoft Excel. Analyses of ANOVA with Holm–Sidak test (Lunardi et al., 2013), Receiver Operator Curve (ROC), and multiple logistic regression were conducted using SigmaPlot 11 (Systat Software). For multiple logistic regression analyses, initial selection of variables with data available in all of the selected subjects in the test cohort was performed according to clinical significance, followed by backward elimination of data (Chang et al., 2015). Six clinical models were constructed, using the same set of the clinical parameters of the test cohort. The equations of the models are listed in Table 2. Models A and B were used to distinguish DN from the non-DN patients (normal control + DM). Models C and D were aimed to stratify DN from DM. Model E and F served to discriminate between normo- and microalbuminuric patients. All decisions on using these tests were taken a priori. In all cases, differences were considered statistically significant at p β 0.05.
Table 2.
Clinical models.
| −AQP5 Model A |
+AQP5 Model B |
−AQP5 Model C |
+AQP5 Model D |
−AQP5 Model E |
+AQP5 Model F |
|
|---|---|---|---|---|---|---|
| Constant | −18.653 | −15,412.986 | −11.032 | −1116.280 | 32.249 | −135.672 |
| X1 | 0.059 | −37.251 | 0.066 | −1.446 | −0.044 | −0.306 |
| X2 | −0.692 | −177.140 | −0.697 | −22.802 | 0.075 | 5.236 |
| X3 | 0.540 | 322.480 | 0.398 | 28.408 | −0.083 | 3.140 |
| X4 | −0.038 | −23.646 | −0.032 | −2.351 | −0.039 | −0.849 |
| X5 | 1.056 | 539.445 | 0.777 | 32.218 | −0.568 | −19.921 |
| X6 | −0.314 | 206.442 | −0.269 | 13.885 | −2.056 | −6.943 |
| X7 | 0.011 | 2.522 | 0.013 | 0.407 | −0.022 | −0.136 |
| X8 | −0.057 | −45.589 | −0.021 | −3.347 | −0.035 | −1.957 |
| X9 | 0.936 | 810.629 | 0.915 | 75.092 | 0.436 | 26.418 |
| X10 | 0.770 | 1157.127 | 0.998 | 86.565 | 1.452 | 20.229 |
| X11 | −0.202 | −568.847 | −0.329 | −44.911 | −1.397 | −26.513 |
| X12 | 1.084 | 0.091 | 0.103 | |||
| X13 | −574.115 | −67.108 | −31.661 |
X1–X13: age, albumin (g/L), total protein, GFR, total glycerides, blood urea nitrogen, uric acid, alanine aminotransferase, fasting blood glucose, direct bilirubin, total bilirubin, urine AQP5/creatinine, and serum AQP5, respectively.
3. Results
3.1. Urine AQP5/creatinine is significantly higher in patients with DN than in normal controls and DM
We first analyzed 84 subjects in a test cohort consisting of normal controls (n = 26) and patients with diabetes mellitus (DM) (n = 25) or DN (n = 33) (Fig. 1A). Table 1 lists their clinical and laboratorial variables. Patients with DN had significantly decreased GFR and serum albumin, compared to patients with DM. Such changes were associated with a significant increase in duration of diabetes, blood urea nitrogen, serum creatinine, globulin, β2-microglobulin, cystatin C, total and direct bilirubin, urinary proteins, and urine albumin-to-creatinine ratio (ACR). Differences in all other parameters including age, body mass index, blood pressure and blood glucose were not significant between the two groups. However, some of these values were significantly higher in DN and DM than in the normal controls.
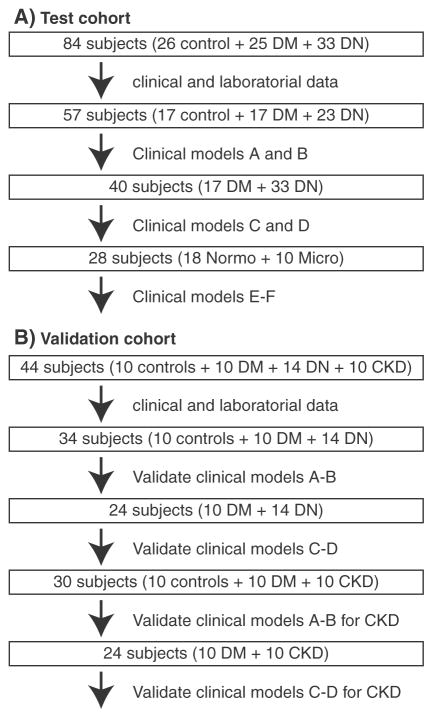
Fig. 1.
Subjects are divided into various populations for model construction and validation. A–B. Diagrams showing the populations of the test and validation cohorts, respectively.
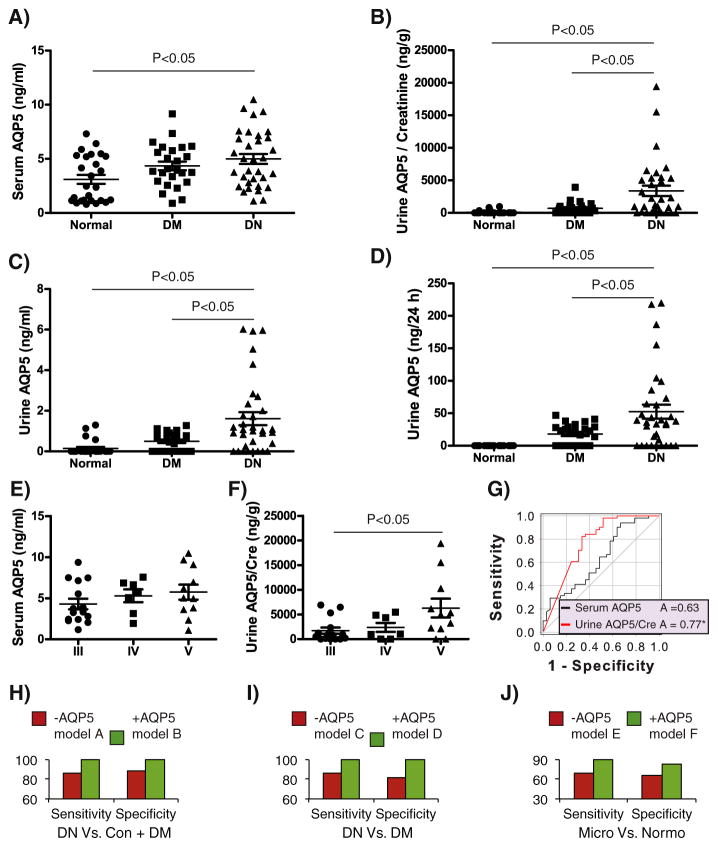
Using an AQP5-specific ELISA, we detected serum AQP5 in all subjects (Fig. 2A). Serum AQP5 displayed a mild increase in DM and a significant increase in DN, compared to normal controls. As shown in Fig. 2B–D, urine AQP5 absolute concentration (ng/ml) was measurable in 4/26 of normal controls, 16/25 of DM, and 25/33 of DN. The mean of urine AQP5/creatinine (ng/g) was significantly higher in DN than in the other two groups, but did not differ significantly between DM and normal controls. Similar results were obtained for urine AQP5 absolute concentration (ng/ml) and the total excretion of AQP5 (ng/24h).
Fig. 2.
Analyses of the test cohort. A–D. Serum and urine AQP5 in 26 normal controls (Normal), 25 DM, and 33 DN were assessed with the AQP5 ELISA kit. It has a detection range of 0.156–10 ng/ml and sensitivity of 0.059 ng/ml. Urine absolute AQP5 concentration (ng/ml), and AQP5/creatinine (ng/mg) and 24 h excretion (ng/24 h) were determined. One-way ANOVA with Holm–Sidak test (Lunardi et al., 2014) was used to determine the statistical significance. E–F. As in A–D except that DN data were reanalyzed and re-plotted according to staging. G. ROC analyses. Shown is the whole sample set (33 DN, and 51 “Non-DN” (26 Normal controls + 25 DM)). H. Shown are clinical models for 57 samples (DN vs. (normal controls + DM), without (−AQP5 model A)) or with urine AQP5/creatinine (+AQP5 model B). I. As in H except that 40 samples (DN vs. DM) were used to generate the clinical models without (−AQP5 model C) or with urine AQP5/creatinine (+AQP5 model D). J. As in I except 18 normoalbumiuric and 10 microalbuminuric patients selected from the 40 DM and DN subjects were used. In all models A–F, the same panel of parameters was used except the absence or presence of urine AQP5/creatinine and serum AQP5.
3.2. Urine AQP5/creatinine is significantly higher in DN stage V than in stage III
The DN group consisted of 15 stage III, 7 stage IV, and 11 stage V patients. There was no significant difference between any stages in serum AQP5 (Fig. 2E), urine absolute AQP5 concentration (ng/ml) and total urine AQP5 excretion (ng/24 h), possibly due to small sample size in each stage (data not shown). Nevertheless, we observed significant higher levels of urine AQP5/creatinine in stage V vs. stage III (Fig. 2F). Hence, urine AQP5/creatinine may increase with progression.
3.3. Urine AQP5/creatinine correlates with many known risk factors of DN
We focused on urine AQP5/creatinine rather than the absolute urine AQP5 concentration (ng/ml) or total excretion of AQP5 (ng/24h) since it is normalized to the internal control creatinine and yielded the highest area under the Receiver Operating Characteristic (ROC) curve (see below). Urine AQP5/creatinine significantly correlated with 20 serum and urine parameters. The correlation coefficients ranged from −0.19 to 0.8. Table 3 lists all of the positive and negative coefficients, respectively. Among the positive ones are blood urea nitrogen, serum creatinine, urine albumin (mg/24 h), urine albumin (mg/ml), and urine albumin/creatinine (mg/g). The negative ones include GFR. Increased urine AQP5 levels are thus apparently associated with increased risk of DN development and impaired kidney function. Serum AQP5 was also positively correlated with serum creatinine (Table 3, see Discussion).
Table 3.
Correlation with urine AQP5/creatinine.
| Parameters | Correlation R |
|---|---|
| Age (yrs) | 0.21* |
| Blood urea nitrogen (mmol/L) | 0.26* |
| Serum AQP5 (ng/ml) | 0.26* |
| Serum creatinine (μmol/L) | 0.30* |
| Serum globulin (g/L) | 0.24* |
| Serum β2-microglobulin (mg/L) | 0.78* |
| Urine albumin (mg/24 h) | 0.64* |
| Urine AQP5 (ng/ml) | 0.88* |
| Urine albumin (mg/ml) | 0.75* |
| Urine albumin/creatinine (mg/g) | 0.68* |
| Urine pH | 0.23* |
| Hematocrit (%) | −0.19* |
| Red blood cells (×1012/L) | −0.35* |
| Serum albumin (g/L) | −0.47* |
| Hemoglobin (g/L) | −0.38* |
| Serum total protein (g/L) | −0.32* |
| Total bilirubin (μmol/L) | −0.22* |
| Direct bilirubin (μmol/L) | −0.27* |
| Urine creatinine (mmol/l) | −0.26* |
| GFR (ml/min/1.73 m2) | −0.36* |
| Serum creatinine (μmol/L)a | 0.33* |
Correlation R with serum AQP5.
p β 0.05.
3.4. Urine AQP5/creatinine improves clinical models in distinguishing DN from normal controls and DM
We combined the normal controls and DM as the “non-DN” group, and performed ROC analysis. The ROC area of serum AQP5 was not significant. In contrast, the ROC area of urine AQP5/creatinine was 0.77 (p β 0.05, Fig. 2G). Therefore, urine AQP5/creatinine may facilitate stratification of DN from normal and DM subjects. Although we tried to collect over 40 clinical parameters from each of the subjects, many of these parameters were not available from numerous subjects, excluding the possibility to include all of the subjects for further analyses. The availability of the related clinical data allowed us to focus on 57 of the 84 subjects. Among them were 17 normal controls, 17 DM, and 23 DN (Fig. 1A). We performed multiple logistic regression analyses based on backward elimination of data (Chang et al., 2015) and constructed a clinical model that excluded ACR to distinguish DN from the non-DN patients. Since ACR is the primary diagnostic parameter, such model also serves to assess the risk factors of DN with micro- and macroalbumia (Inoue et al., 2013). The final model consisted of age, GFR, and serum parameters (uric acid, albumin, total protein, total glycerides, alanine aminotransferase, total bilirubin, direct bilirubin, blood urea nitrogen, and fasting blood glucose). This model (−AQP5 model A) had a sensitivity of 86% and a specificity of 88%, which corresponded to 3/23 false negatives and 4/34 false positives. Addition of AQP5/creatinine and serum AQP5 to create +AQP5 model B increased both the sensitivity and specificity to 100% (Fig. 2H). Next, we excluded the 17 normal controls from the “non-DN” group and focused on DN vs. DM. Using the same panel of parameters, we generated −AQP5 model C and +AQP5 model D. The latter vs. the former improved the sensitivity and the specificity to 1 from 86% and 82%, respectively (Fig. 2I). Similarly, to determine if AQP5 improves discrimination between normo- and microalbuminu-ric patients, we constructed −AQP5 model E and +AQP5 model F by applying the same set of parameters to 28 subjects selected from the DM and DN population. Among them were 18 and 10 normo- and microalbuminuric patients, respectively. Compared to model E, model F increased both the sensitivity and specificity by 20% and 17%, respectively (Fig. 2J). Therefore, our data suggest that urine AQP5/creatinine may improve the clinical models in differentiating DN from normal controls and DM and in differentiating microalbuminuric patients from normoalbuminuric patients.
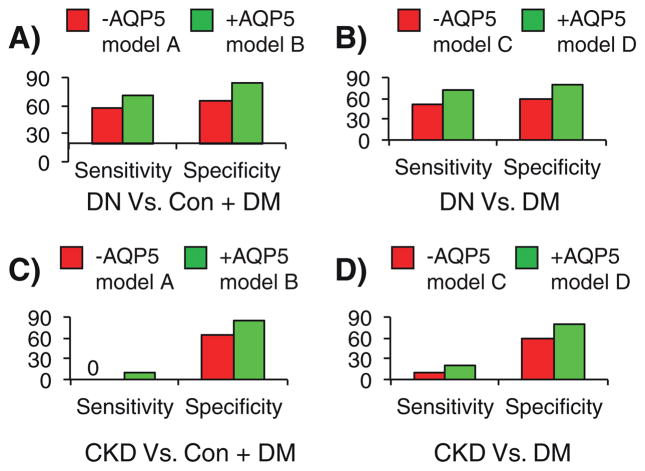
3.5. Urine AQP5/creatinine improves the clinical models in a validation cohort
To test if the increased specificity and sensitivity are not purely related the model fitting and to test if AQP5 is specific for DN or would increase in other forms of chronic and end stage kidney disease, we measured the AQP5 levels and tested the above clinical models in a validation cohort. The cohort consisted of 44 subjects. Among them were 10 normal controls (Con), 10 DM, 14 DN, and 10 CKD of unknown etiology (Fig. 1B). We intended to validate the above clinical models by using the same multiple logistic regression equations described in Table 1 and Fig. 2. In distinguishing DN from Con + DM, the −AQP5 model A resulted in 6/14 false negatives and 7/20 false positives, corresponding to a sensitivity of 57% and a specificity of 65% (Fig. 3A). The +AQP5 model B reduced the false negatives to 4/14 and false positives to 3/20. Hence, addition of AQP5 improved the sensitivity to 71% and the specificity to 85%, increasing the sensitivity by 14% and the specificity by 20% (Fig. 3A). Similarly, the sensitivity of the clinical models differentiating DN from DM was increased from 50% (−AQP5 model C) to 71% (+AQP5 model D). This change was accompanied with a 20% increase in the specificity (Fig. 3B). By replacing DN with CKD, we tested the sensitivity of the −AQP5 models A and C to differentiate CKD from Con + DM or from DM only. These models predicted none and one of 10 CKD subjects to have the disease, respectively. Addition of AQP5 resulted in only one more subject correctly identified (Fig. 3C and D). Hence, AQP5 was apparently less effective in the clinical models differentiating CKD from normal, DM or both.
Fig. 3. Urine AQP5/creatinine improves the clinical models in the validation cohort.
A–D. The clinical models A–D shown in Fig. 2H–I were applied to distinguish DN (A–B) or CKD (C–D) from the controls in the validation cohort. The validation cohort consists of 10 normal controls (Normal), 10 DM, 14 DN, and 10 CKD of unknown etiology.
4. Discussion
Members of water channel family function primarily in the urinary concentrating mechanism and glandular fluid secretion. Emerging evidence suggests that they are also involved in cancer, infection, and swelling of tissues (Verkman, 2012; Verkman, 2011). A selective defect in lacrimal gland AQP5 trafficking is responsible for Sjögren’s syndrome characterized by dry eye and mouth (Tsubota, Hirai, King, Agre, & Ishida, 2001). Elevated AQP5 expression is associated with advanced tumor stage, positive distant metastasis, and/or unfavorable prognosis in several types of cancer (Guo et al., 2013; Watanabe et al., 2009; Yang, Shi, Cheng, & Deng, 2006; Zhang et al., 2010). However, pathological expression of AQP5 in kidney had not been described until our recent report (Wu et al., 2013).
As the closest homolog of AQP2, AQP5 can interfere with AQP2 membrane localization through protein–protein interaction. Such detrimental effect is prevented in normal kidney because of no or little AQP5 expression. The repression occurs in part through DOT1L-mediated H3 K79 methylation of AQP5 promoter (Wu et al., 2013). Under pathological conditions, such as DN, loss of H3 K79 methylation relieves DOT1L-mediated repression. Consequently, AQP5 is upregulated and contributes to polyuria and polyuria-induced kidney damage, in part by impairing AQP2 apical localization (Wu et al., 2013). The molecular mechanism leading to the presence of AQP5 in urine remains obscure. We can speculate three possibilities. First, AQP5 may be secreted into urine directly from tubular cells due to its small size (27 kD). Secondly, urine AQP5 exists as detached AQP5+ tubular cells. The latter is supported by our detection of detached cells expressing AQP5 in the lumen of the tubules (Wu et al., 2013). Finally, the possibility that higher urine levels of AQP5 are partially contributed by higher serum levels cannot be completely ruled out, given the significant correlation between the serum and urine AQP5 levels and between serum AQP5 and serum creatinine. Since serum AQP5 increases along with creatinine, the filtered load may also increase. Consequently, like other low molecular weight proteins whose urinary excretion is increased in renal failure, AQP5 may have increased urinary excretion due to reduced tubular reabsorption. The negative correlation between urine AQP5/creatinine and GFR also suggests that elevated AQP5 is associated with decreased renal function. In addition, circulating AQP5 may be reabsorbed into the tubular cells, resulting in staining of the cells.
Urine AQP5 was also found in 7/36 of normal controls and 23/35 of DM in the two cohorts. These individuals lacked clinical evidence of either obvious kidney disease or significantly impaired GFR. In particular, their albumin excretion was normal. This raises the possibility that AQP5 may serve as an early marker for tubular damage and precede albumin excretion. AQP5 may, thus be added to the list of tubular markers such as kidney injury molecule-1, α1-microglobulin, and retinol binding protein (RBP). These complement the growing list of urine-accumulated tubular epithelial- and glomerular-derived proteins that may have prognostic significance in the context of diabetic renal disease including lipocalin 2, N-acetyl-beta-glucosaminadase, cystatin C, type IV collagen, nephrin, angiotensinogen, L-FABP, the serine proteinase inhibitor PAI-1 and miR-192 (Fiseha, 2015; Jia et al., 2016; Torii et al., 2004). Inclusion of these in patient assessments is likely to provide a more complete evaluation of disease status compared to the more traditional use of a marker of glomerular dysfunction such as albumin (Matheson, Willcox, Flanagan, & Walsh, 2010). Highlighting the utility of these biomarkers is that they provide non-invasive indicators of renal disease onset and/or progression and, at least one (PAI-1) is already an established serum marker and predictor of chronic allograft damage (Chang et al., 2009).
In addition, some CKD patients examined had detectable urinary AQP5, suggesting that application of AQP5 as a potential biomarker may not be limited to DN. Since this is a pilot study with relatively small cohorts, our findings need to be validated in larger and longitudinal studies. Future studies with a large sample size consisting of children and young adults, type 1 diabetic patients, and longitudinal cohort samples are required to further confirm our findings.
5. Conclusion
In summary, our study suggests that urine AQP5/creatinine may have diagnostic and prognostic values as a novel biomarker of diabetic nephropathy and other forms of kidney disease.
Acknowledgments
We thank Paul J. Higgins for reviewing and editing the manuscript. This work was supported by the following grants: National Institutes of Health Grants R01 DK080236 (to W.Z.Z.) and R21 R21DK104073 (to W.Z.Z), and The National Natural Science Foundation of China (NSFC) grants 81173401 and 81470933 (both to Q.L.Z.). The work was initiated when LC pursued his Ph.D. program in W.Z.Z’s lab at the University of Texas Medical School at Houston.
Footnotes
Disclosure: The authors have declared no conflicts of interest.
References
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, Ukpds G. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney International. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- Bhensdadia NM, Hunt KJ, Lopes-Virella MF, Michael Tucker J, Mataria MR, Alge JL, … Arthur JM Veterans Affairs Diabetes Trial study group. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney International. 2013;83:1136–1143. doi: 10.1038/ki.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HR, Yang SF, Lian JD, Lin CC, Wen MC, Chen YT, … Hsieh YS. Prediction of chronic allograft damage index of renal allografts using serum level of plasminogen activator inhibitor-1. Clinical Transplantation. 2009;23:206–212. doi: 10.1111/j.1399-0012.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- Chang CH, Yang CH, Yang HY, Chen TH, Lin CY, Chang SW, … Chen YC. Urinary biomarkers improve the diagnosis of intrinsic acute kidney injury in coronary care units. Medicine. 2015;94:e1703. doi: 10.1097/MD.0000000000001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Silberstein C, Beaulieu V, Pietrement C, Van Hoek AN, Brown D, Breton S. Postnatal expression of aquaporins in epithelial cells of the rat epididymis. Biology of Reproduction. 2006;74:427–438. doi: 10.1095/biolreprod.105.044735. [DOI] [PubMed] [Google Scholar]
- Fiseha T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Biomarker Research. 2015;3:16. doi: 10.1186/s40364-015-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti LM, Jamart J, Dell’omo J, Donckier J. Comparison of urinary excretion of albumin, alpha 1-microglobulin and retinol-binding protein in diabetic patients. Diabetes & Metabolism. 1996;22:324–330. [PubMed] [Google Scholar]
- Gohda T, Tomino Y. Novel biomarkers for the progression of diabetic nephropathy: Soluble TNF receptors. Current Diabetes Reports. 2013;13:560–566. doi: 10.1007/s11892-013-0385-9. [DOI] [PubMed] [Google Scholar]
- Guo X, Sun T, Yang M, Li Z, Li Z, Gao Y. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. BioMed Research International. 2013;2013:206525. doi: 10.1155/2013/206525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm J, Nielsen NV, Hemmingsen L. Retinopathy in type II diabetes mellitus associated with above-normal urinary excretion of RBP. Kidney International. Supplement. 1994;47:S105–S108. [PubMed] [Google Scholar]
- Inoue K, Wada J, Eguchi J, Nakatsuka A, Teshigawara S, Murakami K, … Makino H. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS One. 2013;8:e77118. doi: 10.1371/journal.pone.0077118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Guan M, Zheng Z, Zhang Q, Tang C, Xu W, … Xue Y. miRNAs in urine extracellular vesicles as predictors of early-stage diabetic nephropathy. Journal of Diabetes Research. 2016;2016:7932765. doi: 10.1155/2016/7932765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane CM, Towne JE, Menon AG. Cloning and characterization of murine Aqp5: Evidence for a conserved aquaporin gene cluster. Mammalian Genome. 1999;10:498–505. doi: 10.1007/s003359901030. [DOI] [PubMed] [Google Scholar]
- Lunardi AC, Porras DC, Barbosa RC, Paisani DM, Marques da Silva CC, Tanaka C, Carvalho CR. Effect of volume-oriented versus flow-oriented incentive spirometry on chest wall volumes, inspiratory muscle activity, and thoracoabdominal synchrony in the elderly. Respiratory care. 2014;59(3):420–426. doi: 10.4187/respcare.02665. http://dx.doi.org/10.4187/respcare.02665. [DOI] [PubMed] [Google Scholar]
- Matheson A, Willcox MD, Flanagan J, Walsh BJ. Urinary biomarkers involved in type 2 diabetes: A review. Diabetes/Metabolism Research and Reviews. 2010;26:150–171. doi: 10.1002/dmrr.1068. [DOI] [PubMed] [Google Scholar]
- Merchant ML, Niewczas MA, Ficociello LH, Lukenbill JA, Wilkey DW, Li M, … Klein JB. Plasma kininogen and kininogen fragments are biomarkers of progressive renal decline in type 1 diabetes. Kidney International. 2013;83:1177–1184. doi: 10.1038/ki.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- Mollsten A, Vionnet N, Forsblom C, Parkkonen M, Tarnow L, Hadjadj S, … Groop PH. A polymorphism in the angiotensin II type 1 receptor gene has different effects on the risk of diabetic nephropathy in men and women. Molecular Genetics and Metabolism. 2011;103:66–70. doi: 10.1016/j.ymgme.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney International. 2010;77:57–64. doi: 10.1038/ki.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontuch P, Jensen T, Deckert T, Ondrejka P, Mikulecky M. Urinary excretion of retinol-binding protein in type 1 (insulin-dependent) diabetic patients with microalbuminuria and clinical diabetic nephropathy. Acta Diabetologica. 1992;28:206–210. doi: 10.1007/BF00779000. [DOI] [PubMed] [Google Scholar]
- Pontuch P, Toserova E, Vozar J, Bulas J, Kratochvilova H. 24-h ambulatory blood pressure, daytime and nighttime urinary albumin and retinol-binding protein excretion in type I diabetic patients. Journal of Diabetes and its Complications. 1995;9:234–236. doi: 10.1016/1056-8727(95)80010-c. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. doi: 10.1016/S0140-6736(00)04728-0. [DOI] [PubMed] [Google Scholar]
- Salem MA, el-Habashy SA, Saeid OM, el-Tawil MM, Tawfik PH. Urinary excretion of n-acetyl-beta-D-glucosaminidase and retinol binding protein as alternative indicators of nephropathy in patients with type 1 diabetes mellitus. Pediatric Diabetes. 2002;3:37–41. doi: 10.1034/j.1399-5448.2002.30107.x. [DOI] [PubMed] [Google Scholar]
- Thomas S. Diabetic nephropathy. Elsevier Inc; 2010. [Google Scholar]
- Torii K, Kimura H, Li X, Okada T, Imura T, Oida K, … Yoshida H. Diabetic nephropathy and plasminogen activator inhibitor 1 in urine samplesRinsho Byori. The Japanese Journal of Clinical Pathology. 2004;52:506–512. [PubMed] [Google Scholar]
- Tsubota K, Hirai S, King LS, Agre P, Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Aquaporins at a glance. Journal of Cell Science. 2011;124:2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Aquaporins in clinical medicine. Annual Review of Medicine. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Fujii T, Oya T, Horikawa N, Tabuchi Y, Takahashi Y, … Sakai H. Involvement of aquaporin-5 in differentiation of human gastric cancer cells. The Journal of Physiological Sciences. 2009;59:113–122. doi: 10.1007/s12576-008-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts GF, Powell M, Rowe DJ, Shaw KM. Low-molecular-weight proteinuria in insulin-dependent diabetes mellitus: A study of the urinary excretion of beta 2-microglobulin and retinol-binding protein in alkalinized patients with and without microalbuminuria. Diabetes Research. 1989;12:31–36. [PubMed] [Google Scholar]
- Wu H, Chen L, Zhang X, Zhou Q, Li JM, Berger S, Zhang W. Aqp5 is a new transcriptional target of dot1a and a regulator of aqp2. PLoS One. 2013;8:e53342. doi: 10.1371/journal.pone.0053342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Shi YF, Cheng Q, Deng L. Expression and localization of aquaporin-5 in the epithelial ovarian tumors. Gynecologic Oncology. 2006;100:294–299. doi: 10.1016/j.ygyno.2005.08.054. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Myrup B, Rossing P, Ostergaard PB. Increased tissue factor pathway inhibitor activity in IDDM patients with nephropathy. Diabetes Care. 1996;19:441–445. doi: 10.2337/diacare.19.5.441. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen Z, Song Y, Zhang P, Hu J, Bai C. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. The Journal of Pathology. 2010;221:210–220. doi: 10.1002/path.2702. [DOI] [PubMed] [Google Scholar]