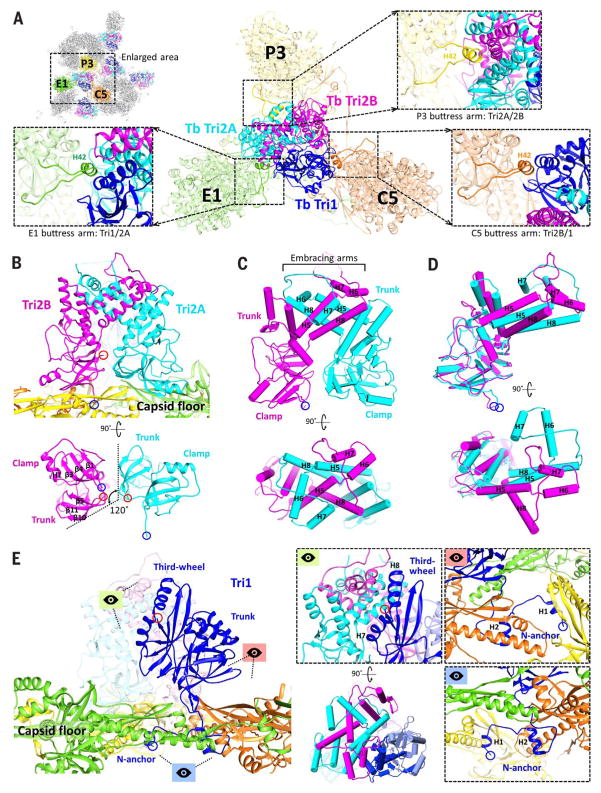
Fig. 6. Triplex structure and interactions with MCP.
(A) Overview of Tb triplex and its surrounding MCPs. Insets show MCP buttress arms that clamp the upper junction regions of the triplex proteins. (B) Tri2A and Tri2B form dimers that interact closely with the capsid floor. Their bottom views reveal similar clamp and trunk domain footprints, albeit rotated 120° about each other. (C) Pipe-and-plank depictions of Tri2 dimer in profile and top views, illustrating the helix bundle formed from the embracing arm domains of Tri2A and Tri2B. (D) Superposing Tri2A and Tri2B reveals nearly identical clamp and trunk domains but highlights conformational differences in their embracing arms. (E) Tri1’s main mass exhibits little contact with the capsid floor compared with Tri2 dimer. Instead, Tri1 secures Tri2 dimer to the capsid through a latch-and-anchor function. Tri1’s third-wheel domain wedges into Tri2’s embracing arms, latching Tri1 to Tri2 dimer (green perspective; the pipe-and-plank depiction shows this at a rotation of 90°). Meanwhile, Tri1’s N-anchor penetrates the capsid floor to anchor the complete triplex to the capsid shell (red and blue perspectives).