Fig. 2.
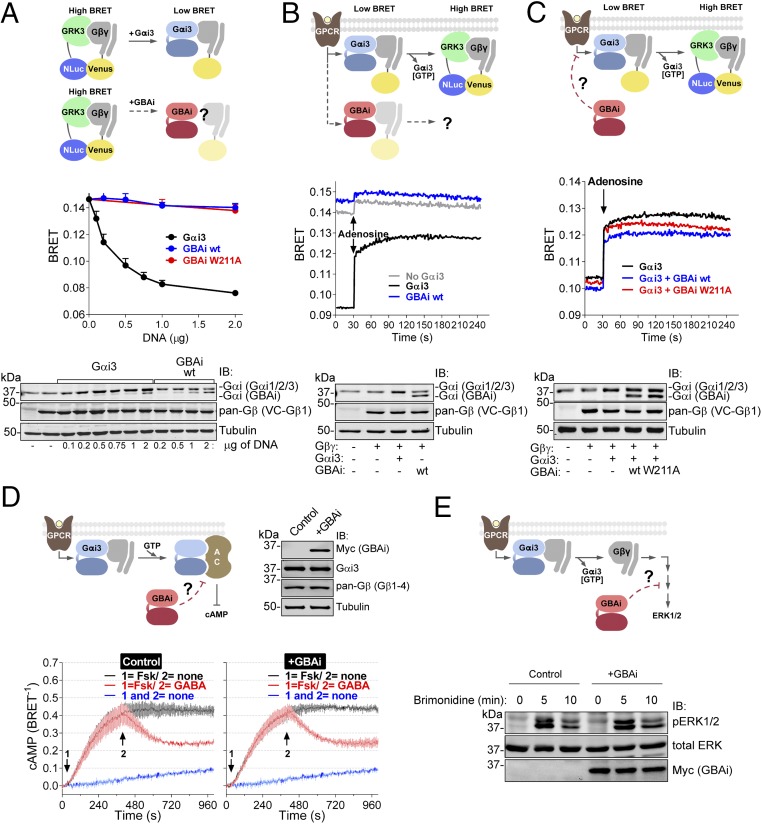
GBAi does not interfere with GPCR-mediated activation of G proteins. (A) GBAi does not associate with Gβγ in mammalian cells as determined by BRET. (Top) Schematic of the BRET assay used to monitor the association of Gαi3 or GBAi with Gβγ. In the absence of Gαi3, Venus-tagged Gβγ (V-Gβγ, BRET acceptor) associates with mas-GRK3ct-NLuc (GRK3, BRET donor) inducing a high BRET signal. Expression of Gαi3 competitively displaces V-Gβγ from mas-GRK3ct-NLuc and decreases BRET. (Middle) HEK293T cells were transfected with the indicated amounts of plasmids encoding for Gαi3, GBAi wt, and GBAi W211A, and equal amounts of the BRET donor (mas-GRK3ct-NLuc) and acceptor (V-Gβγ). BRET was measured under resting (unstimulated) conditions and is presented as mean ± SEM of four independent experiments. (Bottom) Protein expression of Gαi3, GBAi wt, and V-Gβγ was assessed by immunoblotting (IB) with the indicated antibodies. The upper band detected by the Gαi antibody corresponds to exogenous Gαi3 plus endogenous Gαi1, Gαi2, and Gαi3, whereas the lower band corresponds to GBAi. (B) GBAi does not couple to GPCRs in mammalian cells as determined by BRET. (Top) Schematic depicting the BRET assay used to determine the coupling of Gαi3 or GBAi to GPCRs. Under resting conditions, V-Gβγ associates with Gαi3 and BRET is low. Upon GPCR stimulation, the Gαi3:V-Gβγ heterotrimer dissociates and V-Gβγ interacts with mas-GRK3ct-NLuc, leading to an increase in BRET. (Middle) HEK293T cells were transfected with plasmids encoding for Gαi3 (0.5 μg) or GBAi wt (2 μg), along with plasmids for the BRET donor and acceptor (mas-GRK3ct-NLuc and V-Gβγ), as well as for the adenosine 1 receptor. BRET was measured every second. After 30 s of measurement under resting conditions, cells were stimulated with adenosine (10 μM). One representative experiment of three is shown. (Bottom) Protein expression of Gαi3, GBAi wt, and V-Gβγ was assessed by IB with the indicated antibodies. (C) GBAi does not interfere with GPCR-mediated activation of Gαi3 as determined by BRET. (Top) Schematic depicting the BRET experiment used to assess the possible interference of GBAi with Gαi3 coupling to GPCRs. The experimental design is as in B except that GBAi is coexpressed, along with Gαi3 and the rest of the BRET assay components, to test if it could impair the G protein-dependent BRET increase observed upon GPCR stimulation. (Middle) Experiments were carried out exactly as in B except that GBAi (wt or W211A, 2 μg) and Gαi3 (0.5 μg) were expressed simultaneously in the same cells. One representative experiment of three is shown. (Bottom) Protein expression of Gαi3, GBAi, and V-Gβγ was assessed by IB with the indicated antibodies. (D) GBAi does not interfere with GPCR-mediated regulation of cAMP by Gαi. (Top Left) Schematic depicting the experiment used to assess the possible interference of GBAi with GPCR-mediated regulation of cAMP levels. (Top Right) Protein expression of GBAi, Gαi3, and Gβγ was assessed by IB with the indicated antibodies. (Bottom) HEK293T cells were transfected with plasmids encoding for Nluc-EPAC-VV (0.05 μg) and GABABRs (0.2 μg) in the presence (+GBAi) or absence (control) of GBAi wt (2 μg). BRET was measured every 4 s. Forskolin (Fsk, 1 μM) and GABA (1 μM) were added (sequentially) at the indicated times. The blue trace corresponds to unstimulated control cells and is duplicated in both panels as a visual reference of the baseline. Results from three independent experiments are expressed as mean ± SEM. (E) GBAi does not interfere with GPCR-mediated regulation of ERK1/2 by Gβγ. (Top) Schematic depicting the experiment used to assess the possible interference of GBAi with GPCR-mediated regulation of ERK1/2. (Bottom) HEK293T cells were transfected with plasmids encoding for α2A-AR (0.2 μg) in the presence (+GBAi) or absence (control) of GBAi wt (2 μg). Cells were serum-starved overnight and then stimulated with brimonidine (5 μM) for the indicated times. Cell lysates were immunoblotted with the indicated antibodies. One representative experiment of three is shown.