Figure 2.
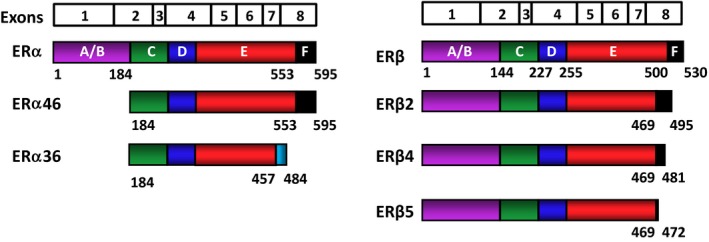
Schematic representation of the estrogen receptor (ER) isoforms. The ERs are encoded by eight exons. The exon boundaries (lines) correspond to the regions of the ERs that are depicted with colored and labeled (A‐F) structural domains. Estrogen receptor α is 595 amino acids long, whereas ERβ is composed of 530 amino acids. Estrogen receptor α46, which is generated by an alternative splicing event, lacks the amino‐terminal A/B region and acts as a competitive inhibitor of ERα. Estrogen receptor α36 is generated from a promoter in the first intron of the ERα gene, together with alternative splicing events that result in a truncated protein with a unique 27 amino‐acid carboxyl‐terminus (light blue) that replaces the last 138 amino acids that are encoded by exons 7 and 8 of wild‐type (WT)‐ERα. Estrogen receptor α36 lacks both activation function (AF)1 and AF‐2. Palmitoylated ERα36 localizes to the plasma membrane and cytoplasm, plays a role in the membrane‐initiated 17β‐estradiol (E2) signaling and adversely affects WT‐ERα‐mediated events. The ERβ isoforms are formed from alternative splicing of exon 8, resulting in carboxyl‐terminally truncated ERβ2, ERβ4, and ERβ5 variants with varying molecular masses. These variants cannot bind ligand and lack AF‐2, but they could adversely affect E2 signaling by heterodimerizing with WT‐ERα or WT‐ERβ when co‐synthesized
