Figure 5.
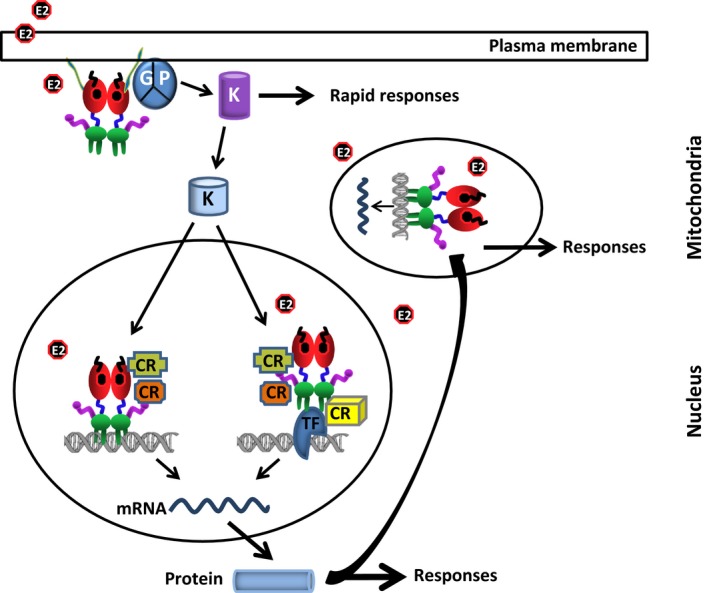
Integrated model of 17β‐estradiol (E2)–estrogen receptor (ER)‐mediated signaling. In the membrane‐initiated signaling, the E2‐bound and palmitoylated (green) ER interacts with a G protein (GP) that results in the activation of kinases, which in turn phosphorylate substrates, including membrane‐based ion channels and secondary messenger systems, leading to rapid cellular responses. The activated kinases also phosphorylate the protein components of the nuclear E2 signaling, including ERs, co‐regulatory proteins, transcription factors (TFs), and chromatin proteins, that result in alterations in responsive gene expression. In the mitochondria, E2‐ER alters the mitochondrial functions by mediating gene expression through a direct interaction with the mtDNA, as well as increasing manganese superoxide dismutase. The mitochondrial functions also are modulated by the nuclear E2‐ERs through the expression of genes, whose protein products are involved in mitochondrial functions. In the nuclear signaling, the ER mediates E2 action with two distinct modes: estrogen response element (ERE)‐dependent and ERE‐independent pathways. The ERE‐dependent signaling route involves the interactions of E2–ER with EREs on DNA and the subsequent regulation of gene expression. The ERE‐independent signaling pathway entails the modulation of responsive gene expression by a direct or indirect, through co‐regulatory proteins (CRs), interaction of E2–ER with transcription factors that are bound to their cognate responsive elements on DNA
