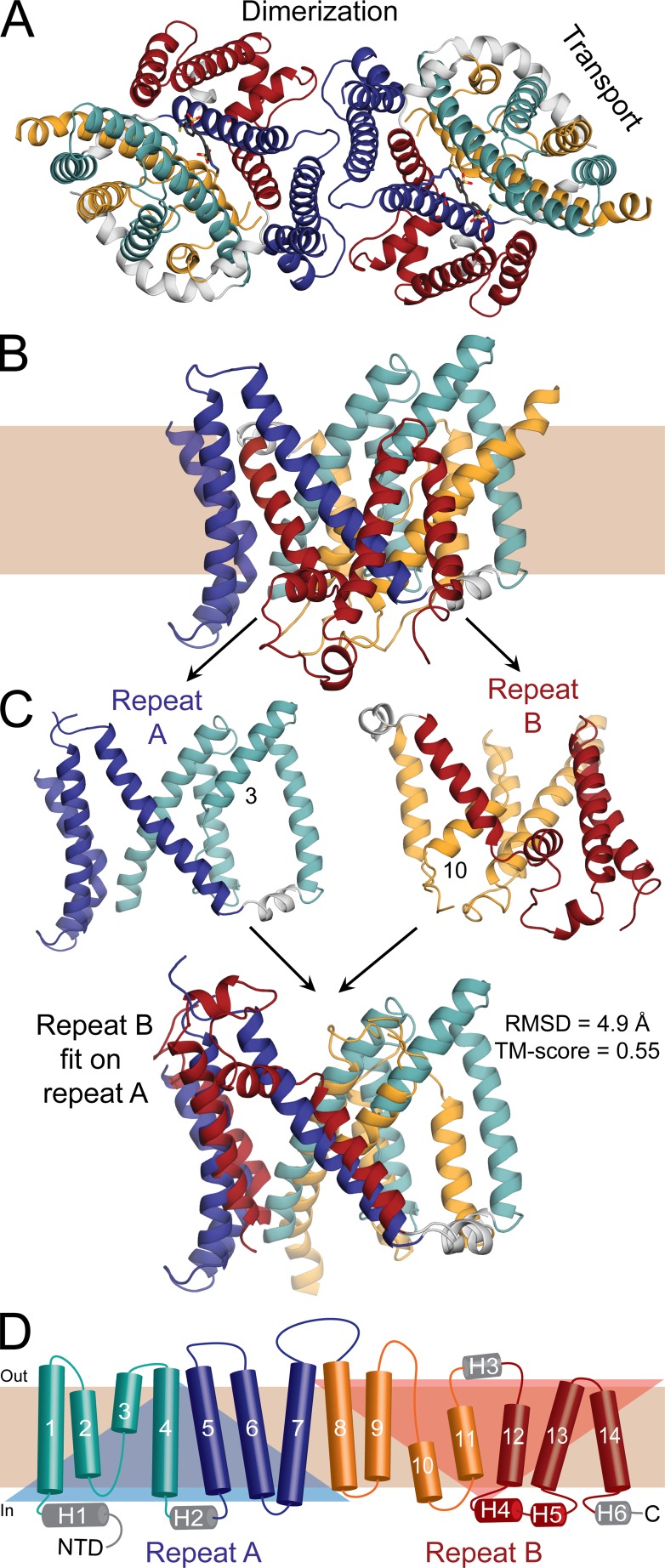
Figure 1.
Structure of the C-terminal domain of human erythrocyte AE1 in an outward-open conformation. (A and B) View of the structure (PDB ID 4YZF) shown as cartoon helices from the extracellular side as a dimer (A) or along the plane of the membrane as a protomer (B). Helices in the transport domain are colored cyan and orange, whereas helices in the dimerization domain are colored red and blue. Peripheral helices are colored gray. (C) Structural repeats A and B, consisting of transmembrane (TM) helices 1–7 (residues 381–635) and TMs 8–14 (residues 636–880), respectively, are shown alone (top) or with repeat B superposed onto repeat A, using TMalign (bottom). The RMSD and TM score resulting from this superposition show that the repeats share the same architecture but display clear differences. The alignment matches 195 residues out of 226. The TM segments that are not continuous helices, TM3 and TM10, are labeled. (D) Transmembrane topology of AE1 highlighting the structural repeats (transparent triangles), and colored according to A. Helices are indicated with cylinders and the extended chain in TM helices 3 and 10 is shown as arrows. Helices outside of the membrane are labeled H1–H6. NTD, N-terminal domain.