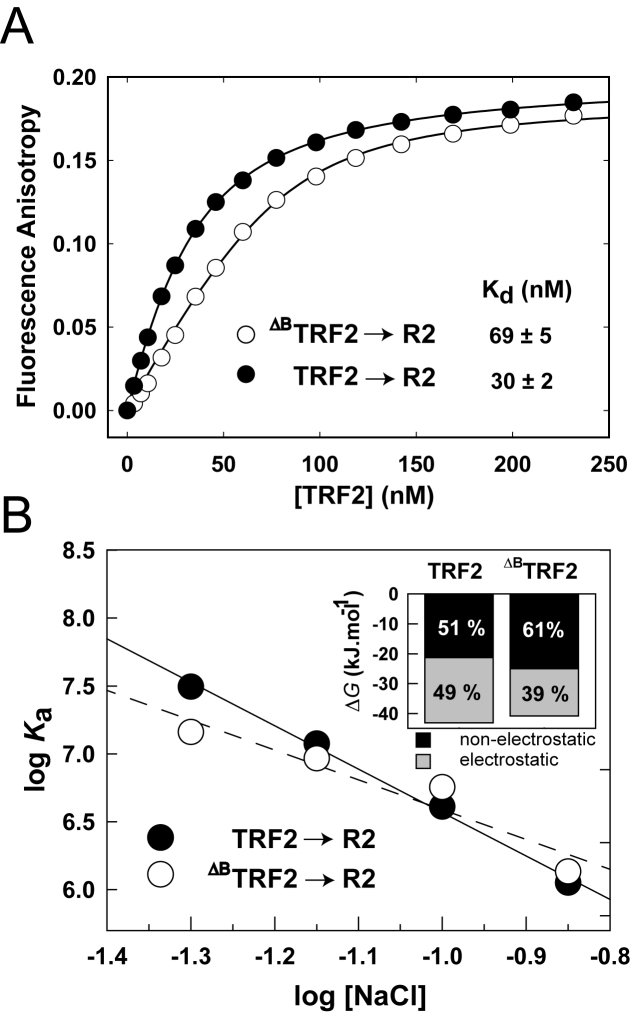
Figure 4.
The B-domain increases TRF2 binding affinity to telomeric DNA via increased electrostatic attraction. (A) TRF2 or ΔBTRF2 (5 μM) was titrated to Alexa Fluor 488 labeled DNA duplex (7.5 nM) in 50 mM sodium phosphate, pH 7.0, 50 mM NaCl at 25°C. Binding isotherms of full-length TRF2 (closed circles) and truncated ΔBTRF2 (open circles) binding to telomeric DNA duplex R2 containing two telomeric repeats were measured by fluorescence anisotropy. (B) The B-domain of TRF2 enlarges the electrostatic attraction to telomeric DNA. The dependence of logarithm of the association constant for binding of TRF2 or ΔBTRF2 to telomeric DNA duplex (7.5 nM) on logarithms of NaCl concentration. NaCl concentration ranged from 50 to 140 mM in 50 mM sodium phosphate buffer pH 7.0. The inset bar graph shows the relative contribution of electrostatic and non-electrostatic interactions to the total free energy of binding of TRF2 or ΔBTRF2 to telomeric DNA.