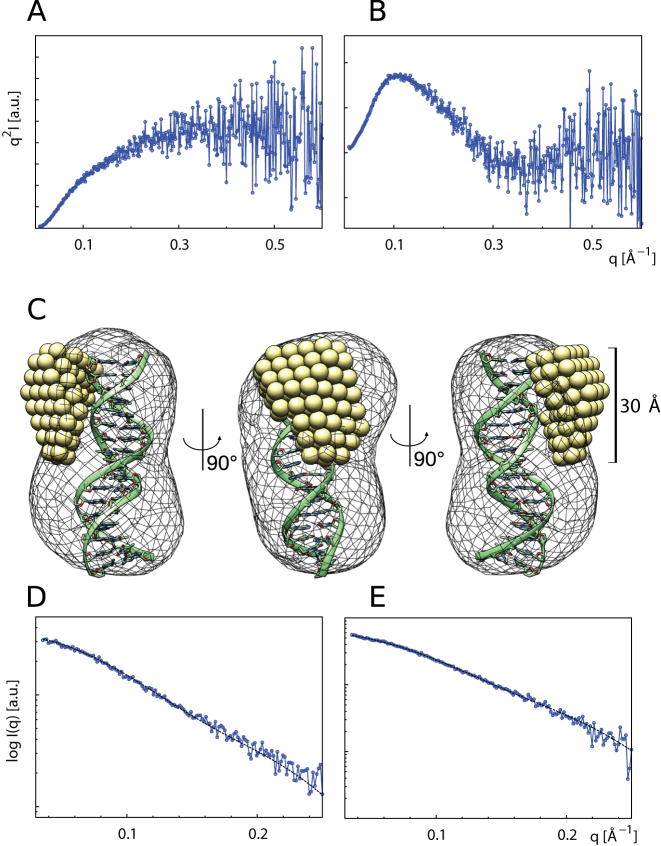
Figure 5.
The B-domain of TRF2 becomes rigid upon binding to telomeric DNA duplex. Kratky plots (I*q2 versus q) of the unbound B-domain (A) and B-domain in complex with telomeric DNA duplex (B). Scattering data from the unbound B-domain (A) exhibit increase characteristic for macromolecules with substantial flexible or unstructured regions, while scattering data from the B-domain–DNA complex (B) display clear minimum characteristic for well-folded compact particles. (C) The typical MONSA model of the B-domain–DNA complex. The DNA phase (mesh) is superimposed with molecular model of telomeric DNA duplex. The protein phase (spheres) illustrates the bound B-domain as a compact body. Fits of simulated scattering to experimental data: (D) DNA phase with final χ2 value 1.22. (E) B-domain–DNA complex phase with χ2 value 1.06.